DOI:
10.1039/C4RA04386C
(Paper)
RSC Adv., 2014,
4, 49400-49414
Intermolecular interactions of CTAB and potential oxidation inhibitors: physico-chemical controlled approach for food/pharmaceutical function†
Received
11th May 2014
, Accepted 24th September 2014
First published on 26th September 2014
Abstract
We report the impact of lipophilic oxidation inhibitors i.e. butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) on cationic surfactant, cetyltrimethylammonium bromide (CTAB) properties in short chain alcohols and their hydroalcoholic solutions. Considering the extensive employment of BHA and BHT in human products, controlled physicochemical approaches were taken into consideration. Principally, density (ρ), ultrasonic sound velocity (u) and viscosity (η) measurements were carried out at specific temperatures T = (298.15, 303.15 and 308.15) K. The physico-chemical parameters apparent molar volume (Vϕ) and apparent molar adiabatic compression (Kϕ,S) were calculated and revealed the existence of hydrophobic interactions whereas, viscosity measurements depicted the region of micellization. Additionally, 1H NMR spectroscopic study was employed to interpret the intermolecular interaction of antioxidants within the micellar structure. 1H NMR analysis suggested the intermolecular interaction especially in the hydrophilic head and interface region of the surfactant with regard to shifting obtained within spectrum.
Introduction
Surfactants are amongst the most commonly utilized products worldwide.1 In particular, nowadays cationic surfactants are widely employed in large quantities as constituents of many pharmaceutical formulations, cosmetics and food products.2–4 In order to improve the applications of antioxidants in modern technologies and their physicochemical evaluation with biological properties, it is necessary to investigate, understand and develop the structure–property relationship in surfactant systems.5 Surfactants having ability to self-aggregate because of distinct hydrophilic and hydrophobic parts within the molecule, it has been observed that structural and chemical nature of both (surfactant and additive) play important role to decide the extent of solubilization, interaction and locus of solubilizate.6
Cetyltrimetylammonium bromide (CTAB); a cationic surfactant has been taken into consideration in the present study. CTAB is widely used in chemical, biochemical, pharmaceutical and industrial fields and moreover posses antibacterial properties too. Many of its physicochemical properties such as CMC and aggregation numbers have been commonly studied in water.7 In literature, a plenty of work has been reported on the transition and self aggregation of the surfactant and bioactive compounds using various techniques.8–14 On the other hand, phenolic compounds are widely studied for their antioxidant properties as well as antimicrobial effect. Oxidation inhibition refers to both the ability to prevent damage from reactive oxygen species (such as through radical scavenging) or to prevent generation of these species (by binding iron).15 Butylatedhydroxy anisole (BHA) and butylatedhydroxy toluene (BHT) stand among the most employed antioxidants in food products, nutraceuticals and cosmeceuticals to attain desired functional effects.16–20 At the moment, the extent to which BHA and BHT can interact in a specific surfactant solution (hydro-alcoholic) at particular concentration is of our main concern and interest.
In context of the mentioned detail and our interest in bio-active compounds,21–24 we intended to measure physico-chemical properties by using different techniques such as density, ultrasonic velocity, viscosity and spectroscopic analysis to reveal the intermolecular interaction of antioxidants and CTAB in short chain alcohols and hydro-alcoholic medium. Three short chain alcohols were chosen i.e. methanol, ethanol and 1-propanol (1 to 3 carbon chain length). Depending on the concentration of these alcohols, they have high diffusion rate through the skin and have been commonly employed in topical formulations.25 These alcohols and hydroalcoholic system (100%, 70% and 30% v/v) were chosen so as to emphasize on the effect of aqueous rich and alcohol rich solution on the system. Recently, there has been proposing new inorganic micelles also that are formed functionalized with various materials that exhibit biological and environment applications.26–29 The chemical structures of BHA, BHT and CTAB have been presented in Chart 1. This manuscript therefore provides insight regarding existing intermolecular interactions and moreover might be helpful to employ as surfactant aided antioxidant in cosmeceutical or food formulations.
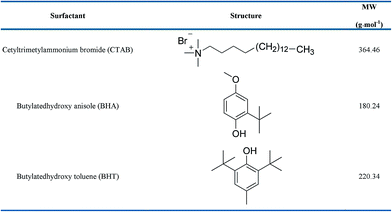 |
| | Chart 1 Chemical structures of CTAB, BHA and BHT. | |
Experimental section
Cetyltrimetylammonium bromide (CTAB) was obtained from sigma and used as such. Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) of AR grade and alcohols i.e. methanol, ethanol and 1-propanol, were obtained from Merck Chemicals. Freshly prepared double distilled water was prepared by double distillation unit obtained from HARCO & Co. and utilized in entire study. In all the experiments, the concentration of BHA and BHT was fixed at 0.03 mol kg−1 and 0.02 mol kg−1, respectively (within the range limit of ADI for average adult i.e. 60 kg) and CTAB concentration ranging from (0.2–1.8) mmol kg−1 (concentration range within the CMC lies). The specification and mass fraction purity of the chemical samples used has been provided in the Table 1.
Table 1 Specification and mass fraction purity of samples used
| Chemical name |
Source |
Mass fraction purity |
| Declared by supplier. |
| Butylated hydroxyanisole (BHA) |
Merck chemicals |
0.99a |
| Butylated hydroxytoluene (BHT) |
Merck chemicals |
0.99a |
| Cetyltrimetylammonium bromide (CTAB) |
Sigma chemical co. |
0.97 |
| Methanol |
Merck chemicals |
0.99 |
| Ethanol |
Merck chemicals |
0.99 |
| 1-Propanol |
Merck chemicals |
0.99 |
DSA-5000 from Anton Paar, a digital high precision instrument was employed in the study for all the density (ρ) and ultrasonic speed of sound (u) measurements at three different temperatures i.e. T = (298.15, 303.15, and 308.15) K. The calibration of the instrument was carried out with de – ionized water (Millipore – Elix system); the conductivity and the pH of water was 1–2 × 10−7 S cm−1 and 6.8–7.0, respectively. The reproducibility of speed of sound and density was ±0.2 m s−1 and ±2 × 10−6 g cm−3 respectively. The data obtained from density and ultrasonic sound velocity was further employed to calculate volumetric and compressibility parameters. The viscosity (η) measurements for various alcoholic/hydroalcoholic solutions were obtained in a calibrated jacketed ubbelohde viscometer using calibrated stopwatch. The viscosity (η) measurements for CTAB in presence of BHA and BHT were determined at three temperatures with an interval of 5 K and accounted for 100%, 70% and 30% (v/v) alcohol (methanol, ethanol and 1-propanol) compositions with water. The approximate flow time of water was 460 seconds at 298.15 K. The viscometer was always placed vertically in a water thermostat having a digital temperature controller to within ±0.02 °C. The samples were kept imperturbable within viscometer for about 10 minutes before every measurement. The precision achieved in viscosity measurement was well within ±0.01%. 1H NMR spectra of the compounds were recorded with Bruker Avance – II 400 NMR spectrometer operating at 400 MHz. The chemical shifts are reported in parts per million (ppm).
Result and discussion
Volumetric and compressibility measurements
The density (ρ) in addition to ultrasonic speed of sound (u) was attained to gain information regarding different interactions with respect to behavior of BHA and BHT in various composite solutions of short chain alcohols [100%, 70% and 30% v/v (methanol, ethanol and 1-propanol)]. The measurements were carried out for CTAB (0.2–1.80) mmol kg−1 containing fixed concentration of BHA (0.03 mol kg−1) and BHT (0.02 mol kg−1) and the study was limited at three specific temperatures at an interval of 5 K (298.15, 303.15 and 308.15) K. The density and ultrasonic velocity data for CTAB in presence of BHA and BHT in methanol compositions have been reported in Table 2 and 3, whereas the data for ethanol and 1–propanol compositions have been provided as ESI (ST1–ST4).† The temperature increment favors the increase of kinetic energy and expansion of volume, therefore, resulting in decrease in density. This also suggests that thermal energy is quite higher than the interaction energy at higher temperatures. On the other hand, decrease in ultrasonic sound velocity with increase in temperature is suggestive of cohesive forces due to existing molecular interactions within the environment. The data obtained from density and ultrasonic sound velocity was further employed to calculate the apparent molar volume (Vϕ) and apparent molar adiabatic compression (Kϕ,S) values. These parameters were calculated using following relation.23,24| |
 | (1) |
| |
 | (2) |
where m (mol kg−1) is the molality of the solution, which was calculated from the molar concentration data using the relation: m = 1/[d/C − M/1000], here m (mol kg−1) stands for the molar concentration and M (g mol−1) for relative molar mass of CTAB, ρ (kg m−3) is the density of the solution, ρo (kg m−3) is the density of the solvent system.κs (TPa−1) and κo (TPa−1) stands respectively for isentropic compressibility of the solution and solvent, respectively and determined by using relation as κs = 1/ρu2.30 In this present investigation, isentropic compressibility values (Table 2 and 3 and ESI†) were found to decrease marginally with increase in surfactant concentration whereas it increases with rise in temperature. Interestingly, isentropic compressibility was found at higher value side within alcohol rich solutions which indicates that hydration in solution system resulting in some kind of solute–solvent interactions. The similar kind of behavior was observed in our previous examination.31 Furthermore, to gain insight with regard to existing interactions between CTAB and BHA or BHT, the behavior of Vϕ and Kϕ,S within the solution system was taken into consideration. The data could not be analyzed in terms of limiting apparent molar volume, (Voϕ) and slope (S*v) values of the Masson's equation ( ), for the reason that Vϕ dependence on CTAB concentration is found to be non – linear which is a contrasting feature with respect to electrolytic solutions.32,33 However, to the best of level, an attempt has been made to derive information with regard to CTAB – BHA/BHT interactions. Errors for Vϕ and Kϕ,S values were calculated and found to lie in the range of ±0.4 m3 mol−1 and ±0.1 m3 mol−1 TPa−1 respectively. All the obtained values of Vϕ and Kϕ,S were found to be positive at all the studied temperatures and concentrations, depicting the existence of hydrophobic interactions and the corresponding data have been reported in Tables 4–7. The variation in Vϕ values for CTAB in presence of BHA in 30% v/v hydro-alcoholic solutions (methanol, ethanol and 1-propanol) is represented in Fig. 1, whilst the BHT trend was in agreement with BHA. From the plot shown in Fig. 1 six pertinent features in the trends of Vϕ for CTAB are as follows:
), for the reason that Vϕ dependence on CTAB concentration is found to be non – linear which is a contrasting feature with respect to electrolytic solutions.32,33 However, to the best of level, an attempt has been made to derive information with regard to CTAB – BHA/BHT interactions. Errors for Vϕ and Kϕ,S values were calculated and found to lie in the range of ±0.4 m3 mol−1 and ±0.1 m3 mol−1 TPa−1 respectively. All the obtained values of Vϕ and Kϕ,S were found to be positive at all the studied temperatures and concentrations, depicting the existence of hydrophobic interactions and the corresponding data have been reported in Tables 4–7. The variation in Vϕ values for CTAB in presence of BHA in 30% v/v hydro-alcoholic solutions (methanol, ethanol and 1-propanol) is represented in Fig. 1, whilst the BHT trend was in agreement with BHA. From the plot shown in Fig. 1 six pertinent features in the trends of Vϕ for CTAB are as follows:
Table 2 Density, ρ (kg m−3), ultrasonic velocity, u (m s−1) and isentropic compressibility, κs (TPa−1) of CTAB (0.2–1.8 mmol kg−1) in water–methanol compositions (% v/v) of 0.03 mol kg−1 BHA over different temperatures ranging from (298.15 to 308.15) K
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| κs TPa−1 10−9. Standard uncertainties in ρ, u and κs are ± 4 × 10−3 kg m−3 ± 0.5 m s−1 and ±0.01 × 10−10 TPa−1. For temperature measurements (T/K), it comes out to be ± 0.01 K. |
| ρ/kg m−3 |
| 0.0 |
788.156 |
784.873 |
772.948 |
840.248 |
828.386 |
818.085 |
883.644 |
871.432 |
859.847 |
| 0.2 |
778.984 |
774.584 |
761.424 |
832.938 |
821.464 |
811.839 |
875.944 |
864.345 |
853.294 |
| 0.4 |
777.132 |
773.132 |
759.353 |
831.012 |
820.147 |
810.783 |
875.111 |
863.673 |
852.455 |
| 0.6 |
775.484 |
772.484 |
758.011 |
831.084 |
819.574 |
809.995 |
874.374 |
862.789 |
851.566 |
| 0.8 |
778.158 |
774.158 |
761.845 |
832.993 |
821.577 |
811.892 |
875.905 |
864.312 |
853.275 |
| 1.0 |
778.545 |
774.545 |
761.794 |
832.912 |
821.503 |
811.815 |
875.835 |
864.293 |
853.196 |
| 1.2 |
777.901 |
774.901 |
761.705 |
832.854 |
821.475 |
811.763 |
875.778 |
864.231 |
853.099 |
| 1.4 |
777.865 |
774.865 |
761.663 |
832.794 |
821.414 |
811.712 |
875.723 |
864.187 |
853.012 |
| 1.6 |
777.796 |
774.796 |
761.597 |
832.722 |
821.354 |
811.665 |
875.686 |
864.146 |
852.934 |
| 1.8 |
777.743 |
774.743 |
761.513 |
832.646 |
821.288 |
811.599 |
875.615 |
864.094 |
852.901 |
| |
| u/m s−1 |
| 0.0 |
1144.71 |
1132.43 |
1121.48 |
1288.46 |
1275.75 |
1262.38 |
1518.55 |
1508.43 |
1495.61 |
| 0.2 |
1137.54 |
1124.54 |
1111.32 |
1284.83 |
1272.43 |
1257.93 |
1512.83 |
1503.74 |
1487.22 |
| 0.4 |
1137.67 |
1124.68 |
1111.45 |
1284.89 |
1272.64 |
1258.12 |
1512.98 |
1503.93 |
1487.38 |
| 0.6 |
1137.84 |
1124.79 |
1111.53 |
1284.95 |
1272.85 |
1258.37 |
1513.22 |
1504.07 |
1487.51 |
| 0.8 |
1137.99 |
1124.88 |
1111.66 |
1285.09 |
1272.91 |
1258.59 |
1513.45 |
1504.18 |
1487.66 |
| 1.0 |
1138.34 |
1124.97 |
1111.78 |
1285.17 |
1273.11 |
1258.74 |
1513.62 |
1504.35 |
1487.79 |
| 1.2 |
1138.51 |
1125.14 |
1111.89 |
1285.18 |
1273.15 |
1258.86 |
1513.75 |
1504.49 |
1487.92 |
| 1.4 |
1138.75 |
1125.29 |
1112.02 |
1285.31 |
1273.28 |
1258.97 |
1513.82 |
1504.62 |
1488.14 |
| 1.6 |
1138.88 |
1125.42 |
1112.17 |
1285.57 |
1273.29 |
1258.99 |
1513.86 |
1504.78 |
1488.39 |
| 1.8 |
1138.89 |
1125.51 |
1112.26 |
1285.66 |
1273.33 |
1259.04 |
1513.98 |
1504.93 |
1488.55 |
| |
| 10−10 κs/TPa−1 |
| 0.2 |
9.921 |
1.021a |
1.063a |
7.272 |
7.518 |
7.784 |
4.988 |
5.116 |
5.298 |
| 0.4 |
9.942 |
1.023 |
1.066 |
7.288 |
7.528 |
7.792 |
4.992 |
5.119 |
5.303 |
| 0.6 |
9.960 |
1.023 |
1.068 |
7.287 |
7.531 |
7.797 |
4.995 |
5.123 |
5.307 |
| 0.8 |
9.923 |
1.021 |
1.062 |
7.269 |
7.512 |
7.776 |
4.984 |
5.114 |
5.295 |
| 1.0 |
9.912 |
1.020 |
1.062 |
7.269 |
7.510 |
7.774 |
4.984 |
5.113 |
5.295 |
| 1.2 |
9.917 |
1.019 |
1.062 |
7.269 |
7.510 |
7.773 |
4.983 |
5.112 |
5.295 |
| 1.4 |
9.914 |
1.019 |
1.062 |
7.268 |
7.509 |
7.773 |
4.983 |
5.111 |
5.294 |
| 1.6 |
9.912 |
1.019 |
1.062 |
7.266 |
7.509 |
7.773 |
4.983 |
5.111 |
5.292 |
| 1.8 |
9.913 |
1.019 |
1.061 |
7.265 |
7.509 |
7.773 |
4.982 |
5.110 |
5.291 |
Table 3 Density, ρ (kg m−3), ultrasonic velocity, u (m s−1) and isentropic compressibility, κs (TPa−1) of CTAB (0.2–1.8 mmol kg−1) in water–methanol compositions (% v/v) of 0.02 mol kg−1 BHT over different temperatures ranging from (298.15 to 308.15) K
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| κs TPa−1 10−9. Standard uncertainties in ρ, u and κs are ± 3 × 10−3 kg m−3 ± 0.3 m s−1 and ± 0.02 × 10−10 TPa−1. For temperature measurements (T/K), it comes out to be ± 0.01 K. |
| ρ/kg m−3 |
| 0.0 |
795.242 |
789.738 |
782.875 |
856.435 |
839.763 |
832.829 |
894.467 |
883.349 |
869.004 |
| 0.2 |
785.353 |
778.439 |
768.345 |
845.632 |
830.292 |
821.293 |
882.839 |
870.754 |
857.353 |
| 0.4 |
783.704 |
777.839 |
767.548 |
844.458 |
829.499 |
820.995 |
880.148 |
869.994 |
857.278 |
| 0.6 |
782.003 |
777.014 |
766.832 |
843.623 |
829.238 |
820.748 |
880.036 |
869.748 |
857.248 |
| 0.8 |
782.249 |
776.949 |
766.786 |
843.784 |
829.112 |
820.292 |
880.374 |
869.685 |
856.984 |
| 1.0 |
784.186 |
776.894 |
766.711 |
843.825 |
829.084 |
820.218 |
880.343 |
869.643 |
856.891 |
| 1.2 |
784.102 |
776.827 |
766.679 |
843.784 |
829.003 |
820.184 |
880.295 |
869.579 |
856.824 |
| 1.4 |
784.058 |
776.764 |
766.607 |
843.766 |
828.974 |
820.132 |
880.211 |
869.515 |
856.776 |
| 1.6 |
783.975 |
776.701 |
766.563 |
843.791 |
828.904 |
820.099 |
880.184 |
869.483 |
856.713 |
| 1.8 |
783.958 |
776.678 |
766.521 |
843.747 |
828.881 |
820.001 |
880.132 |
869.421 |
856.701 |
| |
| U/m s−1 |
| 0.0 |
1167.25 |
1149.05 |
1136.26 |
1295.27 |
1286.43 |
1275.19 |
1531.34 |
1522.81 |
1509.23 |
| 0.2 |
1164.24 |
1146.83 |
1125.23 |
1284.83 |
1280.45 |
1272.89 |
1526.44 |
1515.49 |
1503.24 |
| 0.4 |
1164.39 |
1146.98 |
1125.39 |
1284.89 |
1280.68 |
1272.93 |
1526.48 |
1515.64 |
1503.45 |
| 0.6 |
1164.66 |
1147.13 |
1125.46 |
1284.95 |
1280.69 |
1273.14 |
1526.69 |
1515.75 |
1503.57 |
| 0.8 |
1164.74 |
1147.32 |
1125.75 |
1285.09 |
1280.78 |
1273.29 |
1526.85 |
1515.83 |
1503.73 |
| 1.0 |
1164.76 |
1147.48 |
1125.96 |
1285.17 |
1280.91 |
1273.37 |
1527.12 |
1515.99 |
1503.94 |
| 1.2 |
1164.89 |
1147.61 |
1126.03 |
1285.18 |
1281.05 |
1273.46 |
1527.24 |
1516.15 |
1504.06 |
| 1.4 |
1164.98 |
1147.74 |
1126.26 |
1285.31 |
1281.23 |
1273.74 |
1527.29 |
1516.26 |
1504.28 |
| 1.6 |
1165.05 |
1147.78 |
1126.54 |
1285.57 |
1281.39 |
1273.88 |
1527.38 |
1516.32 |
1504.39 |
| 1.8 |
1165.27 |
1147.93 |
1126.73 |
1285.66 |
1281.55 |
1273.97 |
1527.41 |
1516.33 |
1504.44 |
| |
| 10−10 κs/TPa−1 |
| 0.2 |
9.394 |
9.767 |
1.027a |
7.164 |
7.346 |
7.515 |
4.861 |
5.000 |
5.162 |
| 0.4 |
9.411 |
9.772 |
1.028 |
7.173 |
7.350 |
7.517 |
4.876 |
5.003 |
5.161 |
| 0.6 |
9.427 |
9.780 |
1.029 |
7.179 |
7.352 |
7.517 |
4.875 |
5.004 |
5.161 |
| 0.8 |
9.423 |
9.778 |
1.029 |
7.176 |
7.353 |
7.519 |
4.872 |
5.004 |
5.160 |
| 1.0 |
9.400 |
9.776 |
1.028 |
7.175 |
7.351 |
7.519 |
4.870 |
5.003 |
5.160 |
| 1.2 |
9.398 |
9.774 |
1.028 |
7.175 |
7.350 |
7.518 |
4.870 |
5.002 |
5.159 |
| 1.4 |
9.398 |
9.773 |
1.028 |
7.174 |
7.349 |
7.515 |
4.870 |
5.002 |
5.158 |
| 1.6 |
9.397 |
9.773 |
1.027 |
7.171 |
7.347 |
7.514 |
4.870 |
5.002 |
5.158 |
| 1.8 |
9.394 |
9.771 |
1.027 |
7.170 |
7.346 |
7.514 |
4.870 |
5.002 |
5.157 |
Table 4 Apparent molar volume (Vϕ) (m3 mol−1) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHA (0.03 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| The experimental uncertainties calculate for Vϕ have been comes out to be ± 0.18 × 104 m3 mol−1. |
| 104 ϕv/m3 mol−1 |
| 0.2 |
5.19 |
5.31 |
5.52 |
4.68 |
4.72 |
4.73 |
4.45 |
4.47 |
4.50 |
| 0.4 |
4.90 |
4.96 |
5.14 |
4.49 |
4.52 |
4.54 |
4.23 |
4.26 |
4.31 |
| 0.6 |
4.81 |
4.82 |
4.99 |
4.38 |
4.44 |
4.48 |
4.16 |
4.20 |
4.25 |
| 0.8 |
4.65 |
4.69 |
4.78 |
4.28 |
4.34 |
4.38 |
4.08 |
4.12 |
4.17 |
| 1.0 |
4.60 |
4.64 |
4.73 |
4.26 |
4.31 |
4.36 |
4.05 |
4.10 |
4.15 |
| 1.2 |
4.59 |
4.60 |
4.70 |
4.24 |
4.30 |
4.34 |
4.04 |
4.08 |
4.13 |
| 1.4 |
4.57 |
4.58 |
4.68 |
4.23 |
4.29 |
4.33 |
4.02 |
4.07 |
4.12 |
| 1.6 |
4.55 |
4.57 |
4.66 |
4.22 |
4.28 |
4.32 |
4.02 |
4.06 |
4.12 |
| 1.8 |
4.54 |
4.56 |
4.65 |
4.22 |
4.27 |
4.32 |
4.01 |
4.06 |
4.11 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
5.31 |
5.33 |
5.44 |
4.62 |
4.62 |
4.64 |
4.27 |
4.28 |
4.32 |
| 0.4 |
4.94 |
4.92 |
4.99 |
4.42 |
4.44 |
4.47 |
4.15 |
4.17 |
4.20 |
| 0.6 |
4.74 |
4.76 |
4.83 |
4.31 |
4.35 |
4.39 |
4.08 |
4.10 |
4.14 |
| 0.8 |
4.62 |
4.65 |
4.72 |
4.24 |
4.29 |
4.33 |
4.01 |
4.04 |
4.08 |
| 1.0 |
4.59 |
4.61 |
4.68 |
4.22 |
4.27 |
4.31 |
3.99 |
4.03 |
4.07 |
| 1.2 |
4.56 |
4.58 |
4.65 |
4.21 |
4.25 |
4.30 |
3.98 |
4.02 |
4.06 |
| 1.4 |
4.54 |
4.56 |
4.63 |
4.19 |
4.24 |
4.29 |
3.97 |
4.01 |
4.05 |
| 1.6 |
4.53 |
4.55 |
4.62 |
4.19 |
4.23 |
4.28 |
3.97 |
4.01 |
4.05 |
| 1.8 |
4.52 |
4.54 |
4.61 |
4.18 |
4.23 |
4.28 |
3.96 |
4.00 |
4.04 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
5.90 |
5.96 |
5.98 |
4.66 |
4.67 |
4.68 |
4.54 |
4.57 |
4.68 |
| 0.4 |
5.13 |
5.17 |
5.20 |
4.38 |
4.41 |
4.43 |
4.25 |
4.28 |
4.35 |
| 0.6 |
4.89 |
4.90 |
4.96 |
4.28 |
4.31 |
4.34 |
4.15 |
4.19 |
4.25 |
| 0.8 |
4.66 |
4.71 |
4.75 |
4.23 |
4.26 |
4.30 |
4.08 |
4.14 |
4.19 |
| 1.0 |
4.58 |
4.62 |
4.67 |
4.21 |
4.23 |
4.27 |
4.05 |
4.11 |
4.16 |
| 1.2 |
4.53 |
4.57 |
4.61 |
4.19 |
4.21 |
4.26 |
4.03 |
4.09 |
4.14 |
| 1.4 |
4.50 |
4.53 |
4.57 |
4.18 |
4.20 |
4.25 |
4.02 |
4.08 |
4.12 |
| 1.6 |
4.47 |
4.50 |
4.54 |
4.17 |
4.19 |
4.24 |
4.01 |
4.07 |
4.11 |
| 1.8 |
4.45 |
4.48 |
4.52 |
4.16 |
4.18 |
4.23 |
4.00 |
4.06 |
4.10 |
Table 5 Apparent molar volume (Vϕ) (m3 mol−1) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHT (0.02 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| The experimental uncertainties calculate for Vϕ have been comes out to be ± 0.17 × 104 m3 mol−1. |
| 104 ϕv/m3 mol−1 |
| 0.2 |
5.20 |
5.36 |
5.71 |
4.84 |
4.85 |
5.06 |
4.66 |
4.79 |
4.82 |
| 0.4 |
4.88 |
4.93 |
5.15 |
4.51 |
4.54 |
4.65 |
4.39 |
4.41 |
4.43 |
| 0.6 |
4.78 |
4.80 |
4.96 |
4.40 |
4.42 |
4.51 |
4.24 |
4.27 |
4.30 |
| 0.8 |
4.68 |
4.71 |
4.85 |
4.32 |
4.36 |
4.45 |
4.15 |
4.20 |
4.24 |
| 1.0 |
4.59 |
4.66 |
4.78 |
4.27 |
4.33 |
4.40 |
4.11 |
4.16 |
4.20 |
| 1.2 |
4.56 |
4.63 |
4.74 |
4.25 |
4.30 |
4.37 |
4.08 |
4.13 |
4.17 |
| 1.4 |
4.54 |
4.61 |
4.71 |
4.23 |
4.28 |
4.35 |
4.06 |
4.11 |
4.16 |
| 1.6 |
4.53 |
4.59 |
4.68 |
4.21 |
4.27 |
4.34 |
4.04 |
4.09 |
4.14 |
| 1.8 |
4.51 |
4.57 |
4.67 |
4.20 |
4.26 |
4.32 |
4.03 |
4.08 |
4.13 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
5.50 |
5.57 |
5.62 |
4.86 |
4.91 |
4.88 |
4.52 |
4.55 |
4.63 |
| 0.4 |
5.00 |
5.07 |
5.08 |
4.53 |
4.55 |
4.56 |
4.22 |
4.26 |
4.33 |
| 0.6 |
4.81 |
4.90 |
4.90 |
4.39 |
4.43 |
4.45 |
4.12 |
4.17 |
4.23 |
| 0.8 |
4.70 |
4.76 |
4.80 |
4.28 |
4.36 |
4.38 |
4.04 |
4.11 |
4.17 |
| 1.0 |
4.64 |
4.69 |
4.74 |
4.24 |
4.32 |
4.34 |
4.01 |
4.07 |
4.13 |
| 1.2 |
4.60 |
4.65 |
4.70 |
4.21 |
4.29 |
4.31 |
3.99 |
4.05 |
4.11 |
| 1.4 |
4.57 |
4.62 |
4.67 |
4.20 |
4.27 |
4.30 |
3.98 |
4.03 |
4.09 |
| 1.6 |
4.55 |
4.60 |
4.64 |
4.18 |
4.25 |
4.28 |
3.96 |
4.02 |
4.08 |
| 1.8 |
4.53 |
4.58 |
4.63 |
4.17 |
4.24 |
4.27 |
3.95 |
4.01 |
4.06 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
6.51 |
6.55 |
6.67 |
5.44 |
5.53 |
5.70 |
5.29 |
5.30 |
5.36 |
| 0.4 |
5.39 |
5.44 |
5.53 |
4.73 |
4.80 |
4.91 |
4.62 |
4.65 |
4.71 |
| 0.6 |
5.04 |
5.07 |
5.15 |
4.49 |
4.56 |
4.65 |
4.36 |
4.41 |
4.49 |
| 0.8 |
4.77 |
4.81 |
4.89 |
4.37 |
4.44 |
4.53 |
4.20 |
4.25 |
4.35 |
| 1.0 |
4.66 |
4.70 |
4.77 |
4.30 |
4.37 |
4.45 |
4.13 |
4.18 |
4.28 |
| 1.2 |
4.58 |
4.63 |
4.70 |
4.25 |
4.32 |
4.40 |
4.08 |
4.14 |
4.23 |
| 1.4 |
4.53 |
4.57 |
4.64 |
4.21 |
4.29 |
4.37 |
4.04 |
4.11 |
4.20 |
| 1.6 |
4.49 |
4.53 |
4.60 |
4.19 |
4.26 |
4.34 |
4.02 |
4.08 |
4.17 |
| 1.8 |
4.45 |
4.50 |
4.57 |
4.17 |
4.24 |
4.32 |
4.00 |
4.06 |
4.15 |
Table 6 Apparent molar adiabatic compression (Kϕ,S) (m3 mol−1 TPa−1) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHA (0.03 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| The experimental uncertainties values calculate for Kϕ,S have been comes out to be 0.05 × 102 m3 mol−1 TPa−1. |
| 102 ϕk/m3 mol−1 TPa−1 |
| 0.2 |
51.47 |
54.24 |
58.73 |
34.01 |
35.49 |
36.84 |
22.18 |
22.89 |
23.85 |
| 0.4 |
48.74 |
50.71 |
54.75 |
32.76 |
34.04 |
35.40 |
21.11 |
21.83 |
22.85 |
| 0.6 |
47.88 |
49.32 |
53.28 |
31.93 |
33.42 |
34.89 |
20.76 |
21.53 |
22.56 |
| 0.8 |
46.14 |
47.88 |
50.74 |
31.14 |
32.58 |
34.04 |
20.31 |
21.08 |
22.06 |
| 1.0 |
45.60 |
47.31 |
50.25 |
30.96 |
32.39 |
33.87 |
20.19 |
20.95 |
21.95 |
| 1.2 |
45.49 |
46.91 |
49.93 |
30.84 |
32.27 |
33.75 |
20.11 |
20.87 |
21.88 |
| 1.4 |
45.29 |
46.71 |
49.68 |
30.75 |
32.18 |
33.66 |
20.05 |
20.82 |
21.82 |
| 1.6 |
45.14 |
46.56 |
49.51 |
30.68 |
32.12 |
33.60 |
20.01 |
20.77 |
21.78 |
| 1.8 |
45.04 |
46.45 |
49.37 |
30.63 |
32.07 |
33.56 |
19.98 |
20.74 |
21.74 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
50.85 |
52.98 |
57.41 |
32.97 |
34.24 |
35.74 |
20.85 |
21.48 |
22.38 |
| 0.4 |
47.39 |
48.95 |
52.56 |
31.59 |
32.92 |
34.44 |
20.28 |
20.93 |
21.77 |
| 0.6 |
45.41 |
47.27 |
50.92 |
30.82 |
32.24 |
33.84 |
19.94 |
20.62 |
21.44 |
| 0.8 |
44.21 |
46.13 |
49.58 |
30.30 |
31.72 |
33.27 |
19.55 |
20.27 |
21.13 |
| 1.0 |
43.86 |
45.71 |
49.15 |
30.12 |
31.55 |
33.13 |
19.46 |
20.19 |
21.05 |
| 1.2 |
43.57 |
45.42 |
48.86 |
30.01 |
31.44 |
33.05 |
19.4 |
20.14 |
20.99 |
| 1.4 |
43.38 |
45.22 |
48.65 |
29.93 |
31.36 |
32.97 |
19.36 |
20.10 |
20.95 |
| 1.6 |
43.25 |
45.08 |
48.49 |
29.87 |
31.30 |
32.92 |
19.34 |
20.07 |
20.92 |
| 1.8 |
43.11 |
44.97 |
48.36 |
29.82 |
31.25 |
32.88 |
19.31 |
20.05 |
20.90 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
48.78 |
50.10 |
52.60 |
29.64 |
30.63 |
31.45 |
20.92 |
21.87 |
22.87 |
| 0.4 |
42.50 |
43.53 |
45.75 |
27.87 |
28.88 |
29.74 |
19.60 |
20.48 |
21.29 |
| 0.6 |
40.55 |
41.30 |
43.67 |
27.23 |
28.26 |
29.16 |
19.15 |
20.06 |
20.76 |
| 0.8 |
38.46 |
39.53 |
41.71 |
26.92 |
27.86 |
28.88 |
18.81 |
19.82 |
20.49 |
| 1.0 |
37.81 |
38.84 |
40.99 |
26.80 |
27.69 |
28.69 |
18.67 |
19.68 |
20.33 |
| 1.2 |
37.38 |
38.37 |
40.51 |
26.66 |
27.57 |
28.58 |
18.57 |
19.59 |
20.23 |
| 1.4 |
37.10 |
38.03 |
40.16 |
26.57 |
27.49 |
28.50 |
18.51 |
19.52 |
20.15 |
| 1.6 |
36.88 |
37.79 |
39.90 |
26.50 |
27.43 |
28.44 |
18.46 |
19.49 |
20.09 |
| 1.8 |
36.70 |
37.60 |
39.71 |
26.45 |
27.39 |
28.39 |
18.42 |
19.45 |
20.05 |
Table 7 Apparent molar adiabatic compression (Kϕ,S) (m3 mol−1 TPa−1) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHT (0.02 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 25 °C |
30 °C |
35 °C |
25 °C |
30 °C |
35 °C |
25 °C |
30 °C |
35 °C |
| The experimental uncertainties values calculate for Kϕ,S have been comes out to be 0.04 × 102 m3 mol−1 TPa−1. |
| 102 ϕk/m3 mol−1 TPa−1 |
| 0.2 |
48.82 |
52.39 |
58.70 |
34.65 |
35.60 |
38.00 |
22.63 |
23.96 |
24.87 |
| 0.4 |
45.91 |
48.20 |
52.93 |
32.35 |
33.37 |
34.93 |
21.39 |
22.07 |
22.86 |
| 0.6 |
45.06 |
46.93 |
51.04 |
31.56 |
32.53 |
33.90 |
20.66 |
21.38 |
22.18 |
| 0.8 |
44.14 |
46.09 |
49.88 |
30.99 |
32.09 |
33.44 |
20.24 |
21.02 |
21.88 |
| 1.0 |
43.14 |
45.58 |
49.20 |
30.67 |
31.81 |
33.11 |
20.02 |
20.80 |
21.67 |
| 1.2 |
42.87 |
45.25 |
48.74 |
30.47 |
31.63 |
32.88 |
19.87 |
20.65 |
21.54 |
| 1.4 |
42.67 |
45.01 |
48.41 |
30.31 |
31.49 |
32.70 |
19.78 |
20.55 |
21.43 |
| 1.6 |
42.54 |
44.83 |
48.14 |
30.18 |
31.39 |
32.58 |
19.70 |
20.47 |
21.36 |
| 1.8 |
42.41 |
44.68 |
47.94 |
30.10 |
31.30 |
32.49 |
19.64 |
20.41 |
21.30 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
50.80 |
52.50 |
54.68 |
33.41 |
34.99 |
36.35 |
21.14 |
21.75 |
23.04 |
| 0.4 |
46.24 |
47.87 |
49.51 |
31.20 |
32.44 |
33.98 |
19.71 |
20.41 |
21.55 |
| 0.6 |
44.46 |
46.30 |
47.82 |
30.19 |
31.61 |
33.14 |
19.28 |
19.95 |
21.06 |
| 0.8 |
43.48 |
44.93 |
46.81 |
29.35 |
31.10 |
32.65 |
18.88 |
19.66 |
20.75 |
| 1.0 |
42.88 |
44.30 |
46.18 |
29.09 |
30.77 |
32.33 |
18.73 |
19.49 |
20.55 |
| 1.2 |
42.47 |
43.88 |
45.76 |
28.90 |
30.56 |
32.13 |
18.63 |
19.37 |
20.43 |
| 1.4 |
42.19 |
43.58 |
45.46 |
28.78 |
30.40 |
31.98 |
18.55 |
19.29 |
20.34 |
| 1.6 |
41.98 |
43.34 |
45.24 |
28.68 |
30.29 |
31.86 |
18.50 |
19.23 |
20.27 |
| 1.8 |
41.81 |
43.17 |
45.07 |
28.60 |
30.20 |
31.78 |
18.45 |
19.18 |
20.22 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
51.20 |
53.26 |
57.22 |
32.67 |
34.64 |
37.01 |
23.29 |
24.34 |
25.35 |
| 0.4 |
42.41 |
44.27 |
47.46 |
28.40 |
30.11 |
31.85 |
20.38 |
21.36 |
22.29 |
| 0.6 |
39.70 |
41.26 |
44.26 |
26.98 |
28.60 |
30.20 |
19.27 |
20.29 |
21.28 |
| 0.8 |
37.50 |
39.09 |
41.91 |
26.26 |
27.84 |
29.38 |
18.48 |
19.51 |
20.60 |
| 1.0 |
36.59 |
38.16 |
40.89 |
25.82 |
27.38 |
28.89 |
18.17 |
19.19 |
20.24 |
| 1.2 |
35.98 |
37.53 |
40.22 |
25.52 |
27.07 |
28.56 |
17.95 |
18.98 |
20.01 |
| 1.4 |
35.54 |
37.09 |
39.74 |
25.31 |
26.85 |
28.33 |
17.80 |
18.83 |
19.85 |
| 1.6 |
35.22 |
36.76 |
39.37 |
25.15 |
26.69 |
28.15 |
17.68 |
18.72 |
19.73 |
| 1.8 |
34.96 |
36.50 |
39.10 |
25.03 |
26.56 |
28.01 |
17.59 |
18.63 |
19.64 |
 |
| | Fig. 1 Apparent molar volume (Vϕ) versus CTAB 30% v/v solution of (a) methanol, (b) ethanol, and (c) 1-propanol containing BHA (0.03 mol kg−1) at T = 298.15 K: ■, 303.15 K: ●, 308.15 K: ▲. | |
(i) with increase in temperature, increase in Vϕ values was attained over the studied temperature range,
(ii) with increase in CTAB concentration, the Vϕ values decreases significantly in all alcoholic solution systems as well as in the presence of antioxidants,
(iii) the observed change in magnitude i.e. curved shape appearance of Vϕ values at certain low CTAB concentrations (Fig. 1) and thereafter the decrease in Vϕ is almost linear reveals absolute dominance of hydrophobic–hydrophobic interactions and also indicating the region of micelle formation. This kind of behavior is in support of earlier report,31
(iv) from one of the well established volumetric properties of surfactant, the Vϕ results, thus also imply that in the concentration region >1.0 mmol kg−1, the Vϕ values are practically independent of CTAB concentration and attributed to region of micellization whereas, for concentration <1.0 mmol kg−1 is due to pre-micellar effect,
(v) the observed anomalous behavior might be associated with some kind of hydrophobic clustering of alcohol molecules,34 and
(vi) the effect of Vϕ values was also reflected in Kϕ,S values, thus both the parameters supporting each other.
In context of the observed behavior of Vϕ and Kϕ,S values in different hydro-alcoholic solutions, it could also be explained that due to addition of surfactant in an aqueous solution containing hydrophobic segments the system becomes thermodynamically favorable for the surfactant to form aggregates with hydrophobic portion of that solvent moiety preferentially. Therefore, this additional hydrophobicity offered by the alcohol molecule may be responsible for the trend obtained.
Viscosity measurements
The present investigation was extended with viscosity measurements with rationale based on competent utility of hydroalcoholic system in topical formulation to disperse active pharmaceutical ingredient (API) and to hold desired viscosity of formulations.35 The co-sphere i.e. the regions of modified solvent system surrounding surfactant/antioxidant molecules may contribute towards a change in the viscosity. Examination of the behavior of viscosity of the solution as a function of concentration, nature of surfactant/antioxidant molecules in a given solvent system may be interpreted in terms of structural changes.
Empirically, it has been found that the viscosities of the surfactant solutions have great thermal dependency. Surfactant solutions have their own characteristics depending on their milieu, however, the viscosity of all the composite solutions decreases with temperature increment. The variation in viscosity values could be well attributed to change in micellization process.36 The viscosity measurements were obtained at same CTAB concentrations, hydroalcoholic compositions and temperatures. The variation in viscosity with regard to BHA and BHT in 30% v/v alcoholic and hydro-alcoholic solutions (methanol, ethanol and 1-propanol) is presented in Table 8 and 9. The viscosity values were found to increase with increase in CTAB concentration and decreases with temperature increment. The obtained trend from the plots depicts the presence of intermolecular interactions. Interestingly, an inflection point was observed at a certain point with respect to CTAB concentration region (∼0.8–1.0) mmol kg−1 and thereafter a linear increment in the viscosity magnitude was obtained. Therefore, this certain point of variation within the region is assumed as region of micelle formation. No second inflection was observed in the studied surfactant concentration range, indicating no structural transition in micelles. The observed increase in the viscosity values with increase in CTAB concentration reveals the existence of cohesive forces due to addition molecules (BHA and BHT) whereas the decrease in viscosity values with temperature increase is attributed to the increased kinetic energy of various constituents in solution. Also, at the molecular level the decrease in viscosity with increase in temperature is due to extra vibration between the particles breaking down the intermolecular forces as well as adhesion between the molecules. So, all the values showed fair agreement with respect to region of micelle formation and were found supportive with regard to BHA and BHT.
Table 8 Viscosity, η (centipoise) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHA (0.03 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| The experimental uncertainties values calculate for η have been come out to be 0.02 centipoise. |
| 0.2 |
1.124 |
1.042 |
0.9855 |
0.9615 |
0.8368 |
0.7712 |
0.7512 |
0.6624 |
0.6022 |
| 0.4 |
1.138 |
1.054 |
0.9982 |
0.9762 |
0.8596 |
0.7825 |
0.7644 |
0.6717 |
0.6146 |
| 0.6 |
1.151 |
1.065 |
1.008 |
0.9823 |
0.8732 |
0.7904 |
0.7774 |
0.6804 |
0.6228 |
| 0.8 |
1.168 |
1.076 |
1.021 |
0.9979 |
0.8974 |
0.8048 |
0.7882 |
0.6915 |
0.6335 |
| 1.0 |
1.182 |
1.089 |
1.038 |
1.024 |
0.9132 |
0.8158 |
0.7978 |
0.7008 |
0.6478 |
| 1.2 |
1.212 |
1.114 |
1.059 |
1.054 |
0.9421 |
0.8399 |
0.8162 |
0.7132 |
0.6637 |
| 1.4 |
1.236 |
1.136 |
1.081 |
1.078 |
0.9686 |
0.8566 |
0.8356 |
0.7374 |
0.6892 |
| 1.6 |
1.258 |
1.154 |
1.101 |
1.099 |
0.9839 |
0.8714 |
0.8598 |
0.7524 |
0.7088 |
| 1.8 |
1.282 |
1.172 |
1.125 |
1.134 |
0.9988 |
0.8968 |
0.8786 |
0.7772 |
0.7296 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
1.458 |
1.282 |
1.166 |
1.325 |
1.202 |
1.024 |
1.214 |
1.131 |
1.002 |
| 0.4 |
1.469 |
1.291 |
1.175 |
1.336 |
1.213 |
1.035 |
1.226 |
1.142 |
1.011 |
| 0.6 |
1.480 |
1.301 |
1.186 |
1.347 |
1.224 |
1.046 |
1.237 |
1.153 |
1.020 |
| 0.8 |
1.491 |
1.311 |
1.194 |
1.356 |
1.235 |
1.055 |
1.249 |
1.164 |
1.031 |
| 1.0 |
1.501 |
1.320 |
1.204 |
1.366 |
1.245 |
1.066 |
1.261 |
1.173 |
1.041 |
| 1.2 |
1.516 |
1.336 |
1.219 |
1.381 |
1.258 |
1.081 |
1.276 |
1.186 |
1.053 |
| 1.4 |
1.530 |
1.349 |
1.244 |
1.397 |
1.271 |
1.099 |
1.292 |
1.199 |
1.066 |
| 1.6 |
1.546 |
1.362 |
1.260 |
1.413 |
1.287 |
1.118 |
1.306 |
1.212 |
1.079 |
| 1.8 |
1.561 |
1.376 |
1.276 |
1.427 |
1.301 |
1.136 |
1.319 |
1.226 |
1.093 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
2.812 |
2.654 |
2.469 |
2.215 |
2.146 |
2.002 |
1.825 |
1.706 |
1.612 |
| 0.4 |
2.826 |
2.664 |
2.481 |
2.228 |
2.161 |
2.015 |
1.839 |
1.712 |
1.614 |
| 0.6 |
2.838 |
2.675 |
2.493 |
2.245 |
2.175 |
2.028 |
1.853 |
1.717 |
1.625 |
| 0.8 |
2.849 |
2.684 |
2.505 |
2.262 |
2.191 |
2.041 |
1.864 |
1.727 |
1.634 |
| 1.0 |
2.862 |
2.694 |
2.517 |
2.279 |
2.205 |
2.054 |
1.877 |
1.739 |
1.646 |
| 1.2 |
2.882 |
2.711 |
2.535 |
2.305 |
2.226 |
2.073 |
1.898 |
1.759 |
1.664 |
| 1.4 |
2.901 |
2.726 |
2.553 |
2.328 |
2.244 |
2.092 |
1.921 |
1.777 |
1.682 |
| 1.6 |
2.916 |
2.741 |
2.571 |
2.352 |
2.265 |
2.109 |
1.942 |
1.796 |
1.701 |
| 1.8 |
2.931 |
2.757 |
2.589 |
2.376 |
2.286 |
2.127 |
1.963 |
1.815 |
1.719 |
Table 9 Viscosity, η (centipoise) of CTAB (0.2–1.8) mmol kg−1 in various compositions of methanol, ethanol and 1-propanol containing BHT (0.02 mol kg−1) over different temperatures ranging from (298.15 to 308.15) Ka
| 103 [CTAB] mol kg−1 |
100% v/v methanol |
70% v/v methanol |
30% v/v methanol |
| 298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
298.15 K |
303.15 K |
308.15 K |
| The experimental uncertainties values calculate for η have been come out to be 0.18 centipoise. |
| 0.2 |
1.254 |
1.145 |
1.066 |
1.024 |
0.8652 |
0.8024 |
0.7828 |
0.6926 |
0.6225 |
| 0.4 |
1.266 |
1.155 |
1.078 |
1.048 |
0.8854 |
0.8134 |
0.7942 |
0.7018 |
0.6328 |
| 0.6 |
1.278 |
1.166 |
1.09 |
1.069 |
0.9051 |
0.8247 |
0.8031 |
0.7108 |
0.6431 |
| 0.8 |
1.291 |
1.176 |
1.102 |
1.088 |
0.9262 |
0.8351 |
0.8124 |
0.7193 |
0.6542 |
| 1.0 |
1.305 |
1.187 |
1.115 |
1.114 |
0.9441 |
0.8488 |
0.8206 |
0.7291 |
0.6654 |
| 1.2 |
1.326 |
1.202 |
1.129 |
1.145 |
0.9694 |
0.8642 |
0.8348 |
0.7463 |
0.6802 |
| 1.4 |
1.343 |
1.221 |
1.146 |
1.176 |
0.9885 |
0.8824 |
0.8491 |
0.7632 |
0.7004 |
| 1.6 |
1.359 |
1.234 |
1.162 |
1.199 |
1.007 |
0.9028 |
0.8664 |
0.7774 |
0.7205 |
| 1.8 |
1.376 |
1.248 |
1.178 |
1.242 |
1.022 |
0.9199 |
0.8823 |
0.7912 |
0.7401 |
| |
| |
100% v/v ethanol |
70% v/v ethanol |
30% v/v ethanol |
| 0.2 |
1.821 |
1.721 |
1.592 |
1.784 |
1.592 |
1.462 |
1.356 |
1.272 |
1.168 |
| 0.4 |
1.833 |
1.730 |
1.604 |
1.798 |
1.606 |
1.475 |
1.368 |
1.284 |
1.181 |
| 0.6 |
1.845 |
1.741 |
1.616 |
1.812 |
1.619 |
1.487 |
1.379 |
1.295 |
1.198 |
| 0.8 |
1.857 |
1.751 |
1.627 |
1.826 |
1.633 |
1.501 |
1.388 |
1.307 |
1.212 |
| 1.0 |
1.869 |
1.760 |
1.639 |
1.840 |
1.647 |
1.514 |
1.399 |
1.319 |
1.225 |
| 1.2 |
1.886 |
1.774 |
1.655 |
1.861 |
1.668 |
1.528 |
1.415 |
1.337 |
1.248 |
| 1.4 |
1.901 |
1.789 |
1.671 |
1.879 |
1.689 |
1.548 |
1.429 |
1.355 |
1.267 |
| 1.6 |
1.917 |
1.803 |
1.686 |
1.899 |
1.708 |
1.567 |
1.446 |
1.374 |
1.288 |
| 1.8 |
1.933 |
1.818 |
1.701 |
1.918 |
1.727 |
1.589 |
1.461 |
1.392 |
1.307 |
| |
| |
100% v/v 1-propanol |
70% v/v 1-propanol |
30% v/v 1-propanol |
| 0.2 |
2.928 |
2.782 |
2.535 |
2.342 |
2.205 |
2.136 |
1.965 |
1.822 |
1.739 |
| 0.4 |
2.939 |
2.793 |
2.546 |
2.352 |
2.216 |
2.145 |
1.976 |
1.834 |
1.748 |
| 0.6 |
2.950 |
2.804 |
2.556 |
2.363 |
2.227 |
2.156 |
1.988 |
1.846 |
1.759 |
| 0.8 |
2.961 |
2.815 |
2.565 |
2.372 |
2.238 |
2.167 |
1.997 |
1.855 |
1.770 |
| 1.0 |
2.972 |
2.826 |
2.576 |
2.384 |
2.247 |
2.178 |
2.008 |
1.864 |
1.781 |
| 1.2 |
2.991 |
2.845 |
2.598 |
2.405 |
2.269 |
2.199 |
2.027 |
1.885 |
1.799 |
| 1.4 |
3.011 |
2.864 |
2.624 |
2.426 |
2.291 |
2.220 |
2.048 |
1.904 |
1.821 |
| 1.6 |
3.031 |
2.883 |
2.643 |
2.456 |
2.313 |
2.241 |
2.069 |
1.925 |
1.842 |
| 1.8 |
3.051 |
2.903 |
2.662 |
2.477 |
2.334 |
2.262 |
2.085 |
1.946 |
1.863 |
1H NMR spectroscopic analysis
There are various ways in which an additive can organize itself within the micellar structure. With regard to the possibilities, additive may reside at the micellar surface or can selectively interact with aliphatic chain of the surfactant most often known to be the interface part or can gets completely incorporated in the hydrophobic core up to certain depth depending on site and the type of existing interaction. To attain more insight on interactions and locus of BHA or BHT within the micellar structure, proton NMR was conducted.37 With regard to the precision of the NMR spectrometer, change of ∼0.01 ppm or greater is accounted as a significant change. The lyophilization technique limited the affect of alcohols in the spectra obtained. The picture provided below (Fig. 2) depicts the structural features and substitutions of cationic surfactant used in the present study i.e. CTAB.
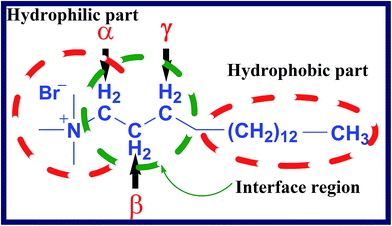 |
| | Fig. 2 The structural features and substitutions of cationic surfactant i.e. CTAB molecule. | |
A close perusal of various proton signals of pure surfactant and the change in the position of these signals in presence of BHA and BHT in various composite solutions. The 1H NMR spectrum of CTAB has been presented in Fig. 3. The intense peaks at ∼0.85 ppm correspond to the aliphatic methyl group, while at ∼3.26, ∼1.65 and ∼1.42 ppm correspond to α, β and γ –(CH2)– respectively. The signal at ∼3.04 ppm correspond to three methyl protons [N+ (CH3)3] with integration of ∼9 protons. The peak for hydrocarbon chain [–(CH2)12–] was obtained at ∼1.24 ppm with integration ∼24 protons. The Fig. 4 and 5 represent the spectrum in presence of BHA and BHT in various composite solutions. The chemical shift was observed in the presence of BHA and BHT revealing significant intermolecular interaction. In particular, up field shift was observed in all the samples. This noticeable up field shift in protons has been shown in Table 10. The α and β –(CH2)– were observed with up field shift of signals ∼0.03 and ∼0.02 ppm respectively, whereas [N+ (CH3)3] and [–(CH2)12–] protons showed ∼0.02 and 0.04 ppm up field shift. The merging of peaks especially, γ –(CH2)– and [–(CH2)12–] was observed which is attributed to micelle growth.38 Moreover, negligible shift of –(CH3)– protons indicated that BHA and BHT no intermolecular interaction within the hydrophobic region of the surfactant. Therefore, at the studied BHA and BHT concentration it was observed that they interact with less hydrophobic region i.e. shell region and cooperating up to interface region.
 |
| | Fig. 3 The 1H NMR spectrum of CTAB molecule. | |
 |
| | Fig. 4 1H NMR spectra of CTAB molecule prepared in (a) water–methanol mixture (b) water–ethanol mixture containing (c) water-1-propanol mixture containing BHA (0.03 mmol kg−1). | |
 |
| | Fig. 5 1H NMR spectra of CTAB molecule prepared in (a) water–methanol mixture (b) water–ethanol mixture containing (c) water-1-propanol mixture containing BHT (0.02 mmol kg−1). | |
Table 10 Proton chemical shifts obtained in CTAB in absence and presence of 0.03 mol kg−1 BHA and 0.02 mol kg−1 BHT in various composite samplesa
| |
–CH3– |
–(CH2)13– |
N+–(CH3)3– |
β–(CH2)– |
α–(CH2)– |
| (–) No proton movement was obtained, C stands for CTAB, A stands for BHA, and T stands for BHT. |
| CAM |
— |
0.02 |
0.04 |
0.02 |
0.03 |
| CTM |
— |
0.03 |
0.03 |
0.02 |
0.03 |
| CAE |
— |
0.02 |
0.04 |
0.02 |
0.03 |
| CTE |
— |
0.02 |
0.04 |
0.02 |
0.03 |
| CAP |
— |
0.02 |
0.04 |
0.02 |
0.03 |
| CTP |
— |
0.03 |
0.04 |
0.02 |
0.03 |
Conclusion
The present paper illustrates the variation in physico-chemical properties of system containing CTAB in presence of BHA and BHT in different alcoholic composite solutions. The obtained results from apparent molal volume (Vϕ) and apparent molar adiabatic compression (Kϕ,S) calculated from density and ultrasonic sound velocity data revealed the existence of hydrophobic interaction. In addition presence of alcohol and hydro-alcoholic solution depicts strong solute–solvent interactions. From 1H NMR spectroscopic analysis; on the basis of chemical shift obtained, it has been hypothesized that intermolecular interaction exists in the hydrophilic region of CTAB cooperating to the interface protons. Therefore, these observations present vital information regarding the micellar delivery of lipophilic molecules. With eminence on the biological diversity of potential synthetic antioxidants and CTAB in alcoholic/hydroalcoholic system, a further development on the BHA/BHT micellar dispersion with standard drug is under progress in our ongoing project.
Acknowledgements
Author V. Bhardwaj and P. Sharma would like to thank DST, New Delhi for financial assistance in the form of major project (Ref. no. SR/FT/CS-59/2009).
References
- C. Tanford, The Hydrophobic Effect: Formation of Micelles and Biological Membranes, J. Wiley., 2nd edn, 1980 Search PubMed
 .
. - G. Ran, Y. Zhang, Q. Song, Y. Wang and D. Cao, Colloids Surf., B, 2009, 68, 106–110 CrossRef CAS PubMed
 .
. - C. J. Drummond and C. Fong, Curr. Opin. Colloid Interface Sci., 1999, 4, 449–456 CrossRef CAS
 .
. - M. Nitschke and S. Costa, Trends Food Sci. Technol., 2007, 18, 252–259 CrossRef CAS PubMed
 .
. - S. Teixeira, C. Siquet, C. Alves, I. Boal, M. P. Marques, F. Borges, J. L. Lima and S. Reis, Free Radicals Biol. Med., 2005, 39, 1099–1108 CrossRef CAS PubMed
 .
. - S. Mehta, S. Chaudhary, K. Bhasin, R. Kumar and M. Aratono, Colloids Surf., A, 2007, 304, 88–95 CrossRef CAS PubMed
 .
. - S. Chauhan, M. Chauhan, D. Kaushal, V. Syal and J. Jyoti, J. Solution Chem., 2010, 39, 622–638 CrossRef CAS
 .
. - T. Chakraborty, I. Chakraborty and S. Ghosh, Langmuir, 2006, 22, 9905–9913 CrossRef CAS PubMed
 .
. - M. Enache and E. Volanschi, J. Pharm. Sci., 2011, 100, 558–565 CrossRef CAS PubMed
 .
. - J. Yang, S. Chen and Y. Fang, Carbohydr. Polym., 2009, 75, 333–337 CrossRef CAS PubMed
 .
. - A. Cid, J. Morales, J. Mejuto, N. Briz-Cid, R. Rial-Otero and J. Simal-Gándara, Food Chem., 2014, 151, 358–363 CrossRef CAS PubMed
 .
. - A. Cid, J. Mejuto, P. Orellana, O. López-Fernández, R. Rial-Otero and J. Simal-Gandara, Food Res. Int., 2013, 50, 143–148 CrossRef CAS PubMed
 .
. - G. Astray, J. Mejuto, J. Morales, R. Rial-Otero and J. Simal-Gándara, Food Res. Int., 2010, 43, 1212–1218 CrossRef CAS PubMed
 .
. - M. Arias, L. García-Río, J. C. Mejuto, P. Rodríguez-Dafonte and J. Simal-Gándara, J. Agric. Food Chem., 2005, 53, 7172–7178 CrossRef CAS PubMed
 .
. - N. R. Perron and J. L. Brumaghim, Cell Biochem. Biophys., 2009, 53, 75–100 CrossRef CAS PubMed
 .
. - J. Sebranek, V. Sewalt, K. Robbins and T. Houser, Meat Sci., 2005, 69, 289–296 CrossRef CAS PubMed
 .
. - H. S. Shah, S. Genier, D. Y. Cheng and B. Patel, US Pat., 5219877, 1993
 .
. - J. J. Kabara, J. Soc. Cosmet. Chem., 1980, 31, 1–10 CAS
 .
. - G. Cevc and U. Vierl, US Pat., 20100086504, 2009
 .
. - J. Kathiriya, B. Sebastian, M. Garg and A. K. Singla, WO Patent 2013124820A1, 2013
 .
. - V. Bhardwaj, P. Sharma, M. Chauhan and S. Chauhan, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.09.008
 .
. - V. Bhardwaj, P. Sharma, M. N. Noolvi, H. M. Patel, S. Chauhan, M. Chauhan and K. Sharma, Thermochim. Acta, 2013, 573, 65–72 CrossRef CAS PubMed
 .
. - P. Sharma, V. Bhardwaj, T. Chaudhary, I. Sharma, P. Kumar and S. Chauhan, J. Mol. Liq., 2013, 187, 287–293 CrossRef CAS PubMed
 .
. - V. Bhardwaj, P. Sharma, M. Chauhan and S. Chauhan, J. Mol. Liq., 2013, 180, 192–199 CrossRef CAS PubMed
 .
. - M. E. Lane, Int. J. Pharm., 2013, 447, 12–21 CrossRef CAS PubMed
 .
. - K. V. Manukyan, B. A. Mason, L. J. Groven, Y.-C. Lin, M. Cherukara, S. F. Son, A. Strachan and A. S. Mukasyan, J. Phys. Chem. C, 2012, 116, 21027–21038 CAS
 .
. - G. Cavallaro, G. Lazzara, S. Milioto, F. Parisi and V. Sanzillo, ACS Appl. Mater. Interfaces, 2013, 6, 606–612 Search PubMed
 .
. - K. Ariga, Q. Ji, M. J. McShane, Y. M. Lvov, A. Vinu and J. P. Hill, Chem. Mater., 2011, 24, 728–737 CrossRef
 .
. - A. Tursunbayeva, V. Abbasov, V. Portnov, H. Ibrahimov, G. Mukhtarova and Y. Lvov, ACS Appl. Mater. Interfaces, 2013, 5, 4464–4471 Search PubMed
 .
. - S. Chauhan, V. Sharma and K. Sharma, Fluid Phase Equilib., 2013, 354, 236–244 CrossRef CAS PubMed
 .
. - V. Bhardwaj, P. Sharma and S. Chauhan, Thermochim. Acta, 2013, 573, 65–72 CrossRef CAS PubMed
 .
. - A. K. Nain, Fluid Phase Equilib., 2007, 259, 218–227 CrossRef CAS PubMed
 .
. - A. K. Nain, Phys. Chem. Liq., 2007, 45, 371–388 CrossRef CAS
 .
. - D. Chandler, Nature, 2005, 437, 640–647 CrossRef CAS PubMed
 .
. - R.-K. Chang, A. Raw, R. Lionberger and L. Yu, AAPS J., 2013, 15, 41–52 CrossRef CAS PubMed
 .
. - C. C. Ruiz, J. Molina-Bolivar, J. Aguiar, G. MacIsaac, S. Moroze and R. Palepu, Langmuir, 2001, 17, 6831–6840 CrossRef CAS
 .
. - P. Sabatino, A. Szczygiel, D. Sinnaeve, M. Hakimhashemi, H. Saveyn, J. C. Martins and P. Van der Meeren, Colloids Surf., A, 2010, 370, 42–48 CrossRef CAS PubMed
 .
. - D. Yu, X. Huang, M. Deng, Y. Lin, L. Jiang, J. Huang and Y. Wang, J. Phys. Chem. B, 2010, 114, 14955–14964 CrossRef CAS PubMed
 .
.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental data of density and speed of sound is presented. See DOI: 10.1039/c4ra04386c |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. 

 ), for the reason that Vϕ dependence on CTAB concentration is found to be non – linear which is a contrasting feature with respect to electrolytic solutions.32,33 However, to the best of level, an attempt has been made to derive information with regard to CTAB – BHA/BHT interactions. Errors for Vϕ and Kϕ,S values were calculated and found to lie in the range of ±0.4 m3 mol−1 and ±0.1 m3 mol−1 TPa−1 respectively. All the obtained values of Vϕ and Kϕ,S were found to be positive at all the studied temperatures and concentrations, depicting the existence of hydrophobic interactions and the corresponding data have been reported in Tables 4–7. The variation in Vϕ values for CTAB in presence of BHA in 30% v/v hydro-alcoholic solutions (methanol, ethanol and 1-propanol) is represented in Fig. 1, whilst the BHT trend was in agreement with BHA. From the plot shown in Fig. 1 six pertinent features in the trends of Vϕ for CTAB are as follows:
), for the reason that Vϕ dependence on CTAB concentration is found to be non – linear which is a contrasting feature with respect to electrolytic solutions.32,33 However, to the best of level, an attempt has been made to derive information with regard to CTAB – BHA/BHT interactions. Errors for Vϕ and Kϕ,S values were calculated and found to lie in the range of ±0.4 m3 mol−1 and ±0.1 m3 mol−1 TPa−1 respectively. All the obtained values of Vϕ and Kϕ,S were found to be positive at all the studied temperatures and concentrations, depicting the existence of hydrophobic interactions and the corresponding data have been reported in Tables 4–7. The variation in Vϕ values for CTAB in presence of BHA in 30% v/v hydro-alcoholic solutions (methanol, ethanol and 1-propanol) is represented in Fig. 1, whilst the BHT trend was in agreement with BHA. From the plot shown in Fig. 1 six pertinent features in the trends of Vϕ for CTAB are as follows:



.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.



