Preparation of highly uniform and crosslinked polyurea microspheres through precipitation copolymerization and their property and structure characterization†
Jinjin Xu,
Hui Han,
Ling Zhang,
Xiaoli Zhu,
Xubao Jiang and
Xiang Zheng Kong*
College of Chemistry & Chemical Engineering, University of Jinan, Jinan, 25002, China. E-mail: xzkong@ujn.edu.cn
First published on 7th July 2014
Abstract
Crosslinked polyurea (PU) microspheres were prepared by precipitation polymerization of isophorone diisocyanate (IPDI) and diethylenetriamine (DETA) in the binary solvent of water–acetone. The influence of polymerization temperature, solvent composition, shaking rate and DETA amount on the microspheres was studied. Highly uniform microspheres were obtained when the polymerization was conducted at 30 °C to 50 °C. A slight decrease in the size of microspheres along with a slightly broadened size distribution was detected with increase in polymerization temperature. With increased water amount in the solvent, the polymerization rate was promoted, the size of the microspheres regularly reduced and the yield regularly enhanced slightly. Microspheres with the best uniformity were observed with water content from 30 wt% to 40 wt%. The results demonstrate that, although PU crosslinking was changed with H2O–acetone ratio in the solvent and polymerization temperature, DETA–IPDI ratio was the most effective means for control of PU crosslinking. The crosslinking was also confirmed by tests on microsphere swellability and light transmittance of the spheres' dispersion in acetic acid. TGA analysis demonstrates that the crosslinked PU was thermally stable. An attempt, based on infrared analysis, to describe the PU structure and its variation with monomer ratio was established. The results were in good agreement with those obtained by theoretical estimation. This work provides a reliable pathway to the preparation of uniform PU microspheres with easily controllable crosslinking.
Introduction
Polymer microspheres with uniform size are of great importance in a great variety of academic and industrial fields, including liquid crystal displays (LCDs),1,2 enzyme immobilization,3 chromatography,4 and drug delivery,5,6 for example. In conventional methods for preparation of these microspheres (suspension polymerization, dispersion polymerization and emulsion polymerization, for example), use of surfactants or stabilizers is compulsory, which is unpleasant in many applications,7 particularly in biological and medical applications. To this end, precipitation polymerization of vinyl monomers, based on free radical mechanism, provides an alternative. A great number of studies have been carried out using different vinyl monomers and/or their combination in different solvents.8–12 Because of the absence of surfactants and stabilizers, monomer concentration in these studies has to be kept at quite low in order to avoid aggregation of the microspheres in growing. In our previous publication, we reported a simple pathway to preparation of polyurea (PU) microspheres,13,14 through the reaction of one single diisocyanate with water. However, the polymers in the microspheres thus prepared were basically linear PU. Note that a prerequisite for the microspheres in most of their applications (LCD, chromatography packing materials) is that their shape or volume must be retained, or in cases where deformation or swelling of the microspheres are expected (as in drug delivery, oil recovery15), they must be controllable. All these mean that there is great demand for the microsphere polymers to be crosslinked in views of academic research and of practical application. In this paper, precipitation copolymerization of isophorone diisocyanate (IPDI) with diethylenetriamine (DETA) is carried out in water–acetone as solvent without any other additives, with expectation to have the microspheres consisting of crosslinked PU. Experimental conditions were optimized in order to achieve the microspheres with uniformed size. A protocol to analysis of the crosslinked PU structure by infrared spectrometry was set up and reliable results were obtained.Experimental
Except otherwise stated, all chemicals used were China domestic products and were used as received. Diethylenetriamine (DETA, AR) and acetone (AR) were purchased from Tianjin Damao Chemicals. Water used was double distilled in the laboratory. Polymerizations were carried out in clean and screw-cap glass bottle of 120 mL. For a typical run, 98 g of H2O–acetone mixture at 30/70 mass ratio was first charged into the reaction bottle, followed by addition of DETA (0.4725 g, 4.59 mmol) and 1.5275 g (6.88 mmol) of isophorone diisocyanate (IPDI, Degussa, German), making DETA–IPDI molar ratio at 2/3 (the ratio of the functional groups NHx/NCO = 1, with x equal to 1 or 2). The bottle was sealed off immediately after IPDI addition, hand shaken for about 10 s to make the mixture homogeneous, and located into a water bath with preset temperature. The polymerization was allowed to proceed for 4 h under quiescent condition, i.e. without any stirring and shaking during polymerization. At end of the procedure, samples were taken and centrifuged for 5 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The microspheres separated were washed twice with acetone prior to drying up at 80 °C for 12 h under vaccum, from which the microsphere yield was calculated. By evaporating the solvent in the supernatant from centrifugation, it was found that there was soluble (unprecipitated) polymer in the supernatant. The yield of this soluble polymer plus that of the microspheres gave always 100% for monomer conversion, thanks to the step-growth polymerization. Runs with varied H2O–acetone mass ratio, polymerization temperature and different reciprocating shaking rates were also conducted. Microspheres were observed under scanning electron microscope (SEM, Hitachi S-2500) using gold coating as commonly known.16 Size (Dn) and size distribution (Dw/Dn) were calculated by counting about 200 microspheres on the SEM pictures. The percentage of crosslinking in PU microspheres was evaluated through Soxhlet extraction using a glass fiber thimble (Safelab-08150, Beijing, China) of 0.3 μm pore size with acetic acid as the reflux solvent (BP 118 °C). Experiment revealed that crosslinked PU microspheres reached their constant weight at 10 h of extraction. The microspheres were also dispersed in acetic acid with 1 wt% concentration at 70 °C for 12 h without shaking and stirring, the solid separated, dried up, and observed under SEM in order to compare the morphology with those before dispersed in acetic acid. The dispersion of the microspheres in acetic acid was also subjected to light transmittance test (at 550 nm wavelengths, Photometer 662, Metrohm) with stepwise temperature increase from 30 °C to 100 °C. The measurement was done after 30 min equilibrium at each test temperature. Thermal gravimetry analysis (TGA) were done for selected samples using Diamond TG/DTA of Perkin Elmer instrument. Fourier Transform Infrared analysis was done using Spectrum GX of Perkin Elmer.
000 rpm. The microspheres separated were washed twice with acetone prior to drying up at 80 °C for 12 h under vaccum, from which the microsphere yield was calculated. By evaporating the solvent in the supernatant from centrifugation, it was found that there was soluble (unprecipitated) polymer in the supernatant. The yield of this soluble polymer plus that of the microspheres gave always 100% for monomer conversion, thanks to the step-growth polymerization. Runs with varied H2O–acetone mass ratio, polymerization temperature and different reciprocating shaking rates were also conducted. Microspheres were observed under scanning electron microscope (SEM, Hitachi S-2500) using gold coating as commonly known.16 Size (Dn) and size distribution (Dw/Dn) were calculated by counting about 200 microspheres on the SEM pictures. The percentage of crosslinking in PU microspheres was evaluated through Soxhlet extraction using a glass fiber thimble (Safelab-08150, Beijing, China) of 0.3 μm pore size with acetic acid as the reflux solvent (BP 118 °C). Experiment revealed that crosslinked PU microspheres reached their constant weight at 10 h of extraction. The microspheres were also dispersed in acetic acid with 1 wt% concentration at 70 °C for 12 h without shaking and stirring, the solid separated, dried up, and observed under SEM in order to compare the morphology with those before dispersed in acetic acid. The dispersion of the microspheres in acetic acid was also subjected to light transmittance test (at 550 nm wavelengths, Photometer 662, Metrohm) with stepwise temperature increase from 30 °C to 100 °C. The measurement was done after 30 min equilibrium at each test temperature. Thermal gravimetry analysis (TGA) were done for selected samples using Diamond TG/DTA of Perkin Elmer instrument. Fourier Transform Infrared analysis was done using Spectrum GX of Perkin Elmer.
Results and discussion
Influence of polymerization temperature
From a previous study on precipitation of IPDI without DETA,14 it is known that the preparation of the microspheres was limited by IPDI solubility in the binary solvent. In 100 g of H2O–acetone at 30/70 (by mass, and the same hereafter), the solubility of IPDI was found to be above 1.8 g at 30 °C. Under this reference, the first set of runs was done using 2.0 wt% of monomers (DETA + IPDI) with DETA–IPDI molar ratio of 2/3 at 30 °C, 40 °C and 50 °C. SEM images of the resulting microspheres are given in Fig. 1, the size and its distribution (Dw/Dn) of the microspheres are listed in Table 1. | ||
| Fig. 1 SEM photos of polyurea microspheres prepared by precipitation polymerization of IPDI and DETA at varied temperature in H2O–acetone solvent of 30/70 mass ratio (A, 30 °C; B, 40 °C; C, 50 °C). | ||
| Polymerization temperature/°C | Turbidness time/min | Dn/μm | Dw/μm | Dw/Dn | Microsphere yield/wt% |
|---|---|---|---|---|---|
| 30 | 8 | 2.76 | 2.79 | 1.007 | 70.28 |
| 40 | 5 | 2.41 | 2.43 | 1.008 | 70.21 |
| 50 | 3 | 2.05 | 2.09 | 1.020 | 71.05 |
From Fig. 1, it is clearly seen that microspheres were well formed at all the tested polymerization temperature with quite uniformed size, particularly at 30 °C and 40 °C (Table 1). A slight decrease in microsphere size was seen with increased polymerization temperature, which may be understood based on the mechanism of this precipitation polymerization.
The mechanism for this process, proposed by Stöver et al. based on an entropy mechanism,9 has been well accepted. Somehow like the particle formation in homogeneous emulsion nucleation,17,18 this entropy mechanism suggests that the particle formation consists of two stages: nucleation and particle growth. Monomers start to polymerize and oligomers are formed, which precipitate out when they reach their critical length where the oligomers becomes insoluble in the solvent. This constitutes the particle nucleation. Increase in polymerization temperature has two opposite effects on the particle formation. One is the increase in the solubility of the oligomers, which would delay their precipitation and the particle nucleation by consequence; at the same time, increase in polymerization temperature may also accelerate the polymerization rate and to promote the oligomers to precipitate out, which will advance the particle nucleation. From a previous study on precipitation polymerization of IPDI without DETA,14 it was found that, by increasing the polymerization temperature, the acceleration of polymerization was predominant over the enhancement of oligomers solubility, which means that more particles are formed by quicker nucleation. Owing to the presence of more polar DETA in the present system, the primitive particles are better stabilized than those formed in the polymerization with only IPDI. Obviously, a greater number of particles will lead to a smaller microsphere size at end of the polymerization at identical monomers concentration. This is in good agreement with the size decrease of the microspheres at higher temperature of polymerization (Fig. 1 & Table 1).
We stress that, since DETA is miscible with the binary solvent, and IPDI amount used here (1.5275 g, 6.88 mmol; DETA 0.4725 g, 4.59 mmol) is within the limit of its solubility, the polymerization system was initially a clear solution, which turned turbid with progress in the polymerization owing to the microsphere formation. Table 1 lists also this time of turbidness which was regularly and slightly shortened with increased polymerization temperature, indicating an advanced particle formation, in accordance with the size variation of the microspheres. In addition, the observation of significantly longer (75 min at 30 °C and 37 min at 50 °C) turbidness time,14 in otherwise the same polymerization except without DETA, suggests that the polymerization rate was largely enhanced with the presence of the amine, DETA.
Influence of H2O–acetone ratio in mixed solvent on microspheres
It is conceivable that the composition of the solvent must have important impact on the formation of the microspheres and their uniformity because not only water molecules may be involved in the polymerization, its amount impose also obvious effect on the particle nucleation since the solubility of monomers and the oligomers depends on the composition of the solvent. Keeping polymerization temperature at 30 °C, a set of polymerizations with varied H2O–acetone ratio in the binary solvent was carried out. Selective SEM pictures of the microspheres thus prepared are given in Fig. 2.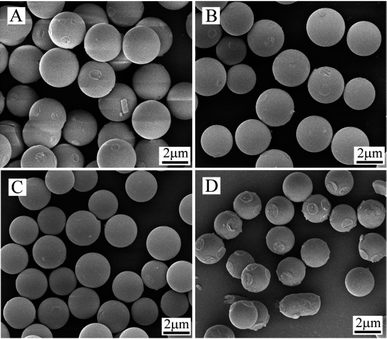 | ||
| Fig. 2 SEM photos of polyurea microspheres prepared by precipitation polymerization of IPDI and DETA at 30 °C in solvent of varied mass ratio H2O–acetone (A, 20/80; B, 25/75; Fig. 1A, 30/70; C, 40/60; D, 50/50). | ||
One can see from Fig. 2 that the size and size distribution of the microspheres were in fact changing in accordance with the change in solvent composition. The granulometric data of the microspheres given in Table 2 show that the turbidness time was sharply shortened with increased H2O amount in the solvent. However, this advancement in turbidity observation is believed to be caused by reduced solubility of the oligomers rather than by the accelerated polymerization rate in this case, albeit water was playing a dual role as co-solvent and a competitive reactant with DETA towards IPDI. This conclusion was based mainly on a well-known fact that the reactivity of isocyanate towards aliphatic amines, particularly the secondary ones, is much higher than that towards water,13,14,19 which means that the probability of water reaction with IPDI must be negligible in the presence of DETA. This conclusion is also supported by the largely extended turbidness time from 8 min (Table 1, 30 °C) to 75 min in the same polymerization without DETA (Quiescent condition, H2O–acetone at 30/70, 30 °C and 2 wt% of monomer).14
| H2O–acetone by mass | Turbidness time/min | Dn/μm | Dw/μm | Dw/Dn | Yield of microsphere/wt% |
|---|---|---|---|---|---|
| 20/80 | 20 | 3.10 | 3.26 | 1.051 | 68.58 |
| 25/75 | 10 | 2.82 | 2.94 | 1.042 | 69.07 |
| 30/70 | 8 | 2.76 | 2.79 | 1.007 | 70.28 |
| 40/60 | 4 | 2.39 | 2.41 | 1.008 | 71.10 |
| 50/50 | <1 | 2.14 | 2.21 | 1.033 | 72.13 |
The corresponding size of the microspheres slightly decreased from 3.10 to 2.14 μm with water content in the mixed solvent increased from 20 wt% to 50 wt% (H2O–acetone ratio from 20/80 to 50/50). This trend of size variation was quite similar to that observed in the runs with increased temperature (Table 1). Nevertheless, the cause is different. The size decrease with increased polymerization temperature (Table 1) was attributed to the acceleration of the polymerization rate; this size decrease with increased water amount in the solvent (Table 2) is believed being caused by formation of a larger number of microspheres owing to reduced solubility of the oligomers in the solvent. At the same time, highly monodisperse microspheres were only obtained for the runs with H2O–acetone ratios at 30/70 and 40/60. Beyond these limits, the uniformity of microspheres was deteriorated with enlarged size distribution (H2O–acetone ratio at 25/75 and 50/50). Similar observation have been reported in the studies on polymerization of IPDI without amine13,14 and on precipitation polymerization of vinyl monomers.12,20 The results were interpreted by arguing that polymerization in the binary solvent with a too high water amount creates a huge amount of primitive particles, leading to their partial or limited aggregation during the process; whereas polymerization in the solvent with a too low water content postpones particle nucleation, resulting in an extended and continuous nucleation. Either one of the two circumstances will cause a broadened size distribution for the microspheres, as one can conclude from the data in Table 2.
It is to note that, while the microsphere yield was almost the same in the runs done at different temperature (Table 1). A slight increase was observed with increased water amount in the binary solvent. This indicates that reducing the solubility of the oligomers by increasing water content in the solvent was more helpful to this yield than by increasing polymerization temperature, although the impact was not important in both cases.
Influence of shaking rate on the polymerization
As one important factor, the shaking rate is also likely to impose great influence on the particle nucleation and growth in precipitation polymerization.14 A set of polymerization was conducted with varied reciprocating shaking rates. The granulometric results and yield of PU microspheres are given in Table 3 (because the similarity of the SEM photos to Fig. 1 & 2, they are not given here but in Fig. S1, ESI†). The results revealed that all the microspheres were quite monodisperse regardless of shaking rates. Comparing the microspheres prepared under quiescent condition, slightly larger size was detected for those prepared under shaking. This is different from the polymerization without use of amine, where more uniform microspheres were observed under quiescent condition.14 This was likely attributable to the presence of DETA, which has dual effect on the polymerization process: (1) polymerization rate was significantly accelerated with the presence of DETA as afore-mentioned, and supported by the turbidness time largely shortened to 8 min (Table 1, 30 °C) from 75 min in the same polymerization without DETA (both conducted at 30 °C under quiescent condition with H2O–acetone at 30/70 and 2 wt% of monomer concentration).14 Shaking is certainly helpful to better monomer diffusion and to impose therefore influence on the polymerization rate and particle growth. This influence was surely lessened with presence of DETA because the polymerization rate was much quicker in this case in comparison to the case without DETA. (2) The stability of the microspheres, throughout the entire process, was enhanced with the presence of DETA because it is of more affinity with the solvent (water–acetone), the impacts of shaking rate on the yield, size and size distribution of the microspheres were therefore largely attenuated as what is seen from the data in Table 3.| Shaking/osc min−1 | Dn/μm | Dw/μm | Dw/Dn | Microsphere yield/wt% |
|---|---|---|---|---|
| 0 | 2.39 | 2.41 | 1.008 | 71.10 |
| 40 | 2.74 | 2.77 | 1.010 | 73.50 |
| 80 | 2.40 | 2.42 | 1.008 | 75.16 |
| 120 | 2.67 | 2.69 | 1.007 | 76.39 |
It is to note that this polymerization proceeds under the mechanism of step polymerization, which means that short oligomers must be abundant at beginning of the polymerization, and that degree of polymerization is function of polymerization time. Determinations of the yields of the microspheres and soluble polymer in the supernatant after centrifugation indicated that monomer conversion was around 100% for all runs. Microsphere yield shown in Tables 1–3 was the portion of the polymers contained in microspheres; the rest of the polymers remained as soluble oligomers in the polymerization medium. The amount of the soluble oligomers was about the same level as what detected in the polymerization without DETA (and so was the microsphere yield). Knowing that DETA (and its copolymer) is of higher water affinity than IPDI, the relative similar yield of microspheres may be owing to the effective crosslinking in the resulted PU, which will promote the yield of microspheres.
Data in Tables 1 and 2 reveal that the yield of the microspheres was hardly changed with polymerization temperature variation from 30 °C to 50 °C; this yield was slightly increased from 68.58% to 72.13% with water content in the solvent increased from 20% to 50%. However, a more perceptible increase was detected with increased shaking rate (Table 3). This may indicate that a better diffusion of the monomers and oligomers was helpful to accelerate the rate of polymerization.
Amount of DETA and PU crosslinking
It is known that, the reactive activity of the proton on a secondary amine, vis-à-via a given isocyanate group, is higher than that in a primary amine.19,21 This is the base to achieve crosslinked PU through the reaction of IPDI with DETA (see ESI, Part 2, Fig. S2†). Actually, it has been confirmed that the PU prepared through the reaction of IPDI with water was uncrosslinked, and crosslinked PU was obtained only when a polyamine was used in the polymerization.16In order to see the effectiveness of DETA in PU crosslinking, effects of solvent composition, polymerization temperature and DETA amount were studied and the percentage of the crosslinked PU was estimated using Soxhlet extraction. The results are listed in Table 4.
| H2O–acetone by mass | DETA–IPDI by mole | NHx/NCO (x = 1, 2) | Temperature/°C | Crosslinked PU/% |
|---|---|---|---|---|
| 20/80 | 2/3 | 1 | 30 | 62.47 |
| 30/70 | 2/3 | 1 | 30 | 75.49 |
| 40/60 | 2/3 | 1 | 30 | 76.49 |
| 40/60 | 2/3 | 1 | 40 | 75.72 |
| 40/60 | 2/3 | 1 | 50 | 74.93 |
| 50/50 | 2/3 | 1 | 30 | 82.96 |
| 40/60 | 2/9 | 1/3 | 30 | 58.89 |
| 40/60 | 2/15 | 1/5 | 30 | 41.84 |
| 40/60 | 1/15 | 1/10 | 30 | 32.61 |
| 40/60 | No DETA | 0 | 30 | 0.0 |
Data in Table 4 indicate that, with increased H2O content in the solvent (data lines 1, 2, 3 and 6), the crosslinked portion in PU microspheres was gradually increased from 62.47% to 82.96%; whereas with increased polymerization temperature from 30 °C to 50 °C (data lines 3, 4 and 5), it was slightly decreased from 76.49% to 74.93%. While the solvent composition and polymerization temperature entrained perceptible but limited changes in the crosslinking of the microspheres (along with deteriorated uniformity of the microspheres as shown earlier, Tables 1 and 2); the amine amount (or DETA–IPDI ratio) was seen as the most effective means to control the crosslinking. The data in Table 4 (lines 3, 7, 8, 9) show that the portion of the crosslinked PU dropped from 76.49% to 32.61% by changing DETA–IPDI molar ratio from 2/3 (NHx/NCO = 1) to 1/15 (NHx/NCO = 1/10).
Solubility and light transmittance of PU microspheres in acetic acid
Through Soxhlet extraction, it was confirmed that the highly uniform microspheres were effectively consisting of crosslinked PU. Here below the study is focusing on the property characterization of the crosslinked microspheres. The properties are compared with those of the microspheres prepared without use of amine, assumingly consisting of linear PU.First test was carried out by dispersing 1.0 g of the PU microspheres into 99 g of acetic acid by mechanical stirring. Two samples were thus prepared, one with the PU prepared by IPDI reaction with water in H2O–acetone binary solvent (H2O–acetone at 40/60), and another in the same solvent but with presence of DETA (DETA–IPDI molar ratio at 2/3, i.e. NHx/NCO = 1). From the data in Table 4, it is known that the former sample was consisting of linear PU, and the latter of crosslinked PU of about 76.5%. The dispersions of the microspheres were subjected to temperature increase by 10 °C from 30 °C to 100 °C. Light transmittance of the dispersion was determined after an equilibrium of 30 min at each temperature.
The results, given in Table 5, demonstrate that light transmittance of the dispersion done with the PU prepared without DETA reached 100% at 50 °C, indicating the PU microspheres were full dissolved in acetic acid, forming a homogeneous solution of linear PU. In contrast, the light transmittance of the dispersion with the PU prepared with DETA never reached 2.0%, even with the temperature going up to 97 °C, indicating the PU microspheres were insoluble in acetic acid due to high crosslinking, in good agreement with the results obtained from Soxhlet extraction (Table 4).
| Temperature (°C) | 30 | 40 | 50 | 70 | 90 | 97 |
|---|---|---|---|---|---|---|
| Transmittance (%) of dispersion of PU prepared without DETA | 72.7 | 97.1 | 100 | 100 | 100 | 100 |
| Transmittance (%) of dispersion of PU prepared with DETA | 1.5 | 1.5 | 1.6 | 1.6 | 1.7 | 1.7 |
The same two dispersion samples, one prepared with the microspheres of linear PU and another with the crosslinked PU (both at 1.0 wt% of microsphere in acetic acid), were kept at 70 °C for 12 h. The former became a clear solution and the latter remained a turbid dispersion. For the linear PU solution, PU polymer was recovered by evaporation of acetic acid; and for the sample with crosslinked PU, the polymer was recovered by centrifugation. The polymers thus obtained were examined under SEM (Fig. 3). In fact, PU polymer recovered from the solution remained at the bottom of the glass container and a disc formed PU block was obtained upon solvent removal. Taken out with a spatula, there were no any microspheres at all as shown in Fig. 3A; in contrast, the crosslinked PU microspheres prepared with DETA remained as is after being recovered from its dispersion in acetic acid followed by centrifugation as seen in Fig. 3B. This information provides supplementary support to the effectiveness of PU crosslinking by copolymerization of IPDI with DETA.
 | ||
| Fig. 3 SEM photos of PU microspheres prepared by precipitation polymerization of IPDI without (A) and with DETA (B, DETA–IPDI = 2/3 by mole) after dispersed in acetic acid for 12 h at 70 °C. | ||
Thermal properties of PU microspheres
DSC test was also carried for a sample prepared with DETA–IPDI at 2/3 (moles), with the temperature scanning from 30 °C to 250 °C at a heating rate of 5 °C min−1 under nitrogen atmosphere using a Mettler-Toledo instrument (DSC 822). No obvious Tg transition was observed. Similar results have been observed in a number of studies on polyureas or polyurethane22,23 and has been interpreted by the heavy presence of hydrogen bonding, which makes most of the polymer segments “frozen” with partial crystallinity, and their free movement is severely restricted till the temperature of their degradation. No valuable information was drawn. The samples were then subjected to TGA test. The results (ESI Fig. S3†) revealed that, likely due to presence of the moisture, a slight weight loss was detected around 100 °C for the linear (prepared by reacting IPDI with H2O) and the crosslinked (prepared by copolymerization of IPDI and DETA) samples. For the linear sample, it remained stable up to about 280 °C, 5% mass loss was seen at 330 °C, 10% at 336 °C, and 50% of the sample was degraded at 355 °C, only about 3% was remained at 450 °C and thereafter. As to the crosslinked sample, its thermal stability was slightly lower. 5% mass loss was seen at 298 °C, 10% at 318 °C, about 20 °C advanced in comparison with the linear PU prepared without DETA. Nevertheless, 50% of the sample loss was observed exactly at the same temperature, i.e. 355 °C, as that in linear PU. And about 4% of the sample remained at 450 °C and thereafter. This is the common behavior for polyurea materials.16,24Infrared spectrometry
It has been shown that linear PU was obtained by IPDI reaction with H2O, and that PU of different crosslinking was synthesized by polymerization of IPDI with varied DETA amount. However, to find a reliable means for the characterization of the microstructure in these PU's was difficult because the crosslinked PU was not soluble (the linear PU was also insoluble in common solvents13). Solid state NMR did not provide reliable results owing to, most likely, the intensive presence of hydrogen bonding, impeding to have clear resonance signals. Great effort was therefore focused on FTIR analysis of the samples. The corresponding spectra are displayed in Fig. 4. | ||
| Fig. 4 FTIR spectra of PU microspheres prepared by copolymerization of IPDI with water (A) and with DETA at different DETA–IPDI molar ratio (B, 2/3; C, 2/9; D, 2/15; E, 1/15). | ||
The absorption peaks were assigned as following: 3360 cm−1, stretching vibration of NH; 2953 cm−1, 2907 cm−1 and 1455 cm−1 (not marked in Fig. 4), stretching vibration of CH in methyl, methylene and methine; 2264 cm−1, stretching vibration of isocyanate (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1647 cm−1, stretching vibration of carbonyl (–C
O); 1647 cm−1, stretching vibration of carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in urea group; 1558 cm−1, bending vibration of NH in urea group; 1455 cm−1 (not marked in Fig. 4), bending vibration of CH in CH3 and CH2CH2 groups; 1366 cm−1 and 1384 cm−1 (not marked in Fig. 4), bending vibration of CH in CH3 groups with 2 methyls attached on a same carbon atom (on IPDI hexagon ring); 1306 cm−1, stretching vibration of CH; 1240 cm−1, stretching vibration of C–N in urea group (–NHCONH–); 868 cm−1, deformation vibration of C–H; 768 cm−1, out-plane bending vibration of CH in –CH2CH2– group; 650 cm−1 bending vibration of C–N in urea group (–NHCONH–). 2361 cm−1 was believed to be owing to the presence of CO2 in the sample. Thus all the characteristic groups in this PU have their corresponding absorptions peaks.
O) in urea group; 1558 cm−1, bending vibration of NH in urea group; 1455 cm−1 (not marked in Fig. 4), bending vibration of CH in CH3 and CH2CH2 groups; 1366 cm−1 and 1384 cm−1 (not marked in Fig. 4), bending vibration of CH in CH3 groups with 2 methyls attached on a same carbon atom (on IPDI hexagon ring); 1306 cm−1, stretching vibration of CH; 1240 cm−1, stretching vibration of C–N in urea group (–NHCONH–); 868 cm−1, deformation vibration of C–H; 768 cm−1, out-plane bending vibration of CH in –CH2CH2– group; 650 cm−1 bending vibration of C–N in urea group (–NHCONH–). 2361 cm−1 was believed to be owing to the presence of CO2 in the sample. Thus all the characteristic groups in this PU have their corresponding absorptions peaks.
Further analysis on the FTIR spectra were carried out in order to elucidate some detailed information on chemical structures of PU. It is well known that absorption peak in FTIR is a reflection of a chemical group, and the peak intensity is closely related to the quantity of the chemical group presented in the sample. Just like in NMR analysis to get the composition of a chemical substance, the relative amount of a chemical group can be also estimated from the height of FTIR peak (when very sharp and asymmetric) or peak area through comparison with the peak height or area of another given chemical group.24–26 Based on this point, one can in principle do an estimation on the proportion of two given chemical groups in a tested sample, and therefore on the variation of the composition of PU if the given groups are appropriately chosen. Obviously, the peaks for the chosen groups to be analyzed for this purpose must be well separated with intensive absorption.
The peak at 3360 cm−1 in Fig. 4 is well recognized as the one by stretching vibration of –NH– and –NH2 (denoted as NHx for simplicity hereafter) and well separated from the rest. Those at 2953 cm−1 and 2907 cm−1 were assigned to stretching vibration of CH in methyl (–CH3), methylene (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) and in methine (
CH2) and in methine (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) (denoted as CHx for simplicity hereafter). In the present case for PU, –NH– is most likely present only in urea group (–NH–CO–N
CH) (denoted as CHx for simplicity hereafter). In the present case for PU, –NH– is most likely present only in urea group (–NH–CO–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), and is formed exclusively by the reaction of isocyanate with primary amine (those in DETA or in situ formed as depicted in Fig. S2, ESI†) since it is known that the secondary amine (–NH–) in DETA is more active than the primary amine in regard to the reaction with isocyanate.19,21 –NH2 has two different origins, one is that present in DETA, another is the primary amine in situ formed through the reaction of IPDI with H2O. Whereas for the CHx in PU, they are all brought about from the monomers (DETA and IPDI), and kept unchanged before and after the polymerization because they are not involved in the polymerization.
), and is formed exclusively by the reaction of isocyanate with primary amine (those in DETA or in situ formed as depicted in Fig. S2, ESI†) since it is known that the secondary amine (–NH–) in DETA is more active than the primary amine in regard to the reaction with isocyanate.19,21 –NH2 has two different origins, one is that present in DETA, another is the primary amine in situ formed through the reaction of IPDI with H2O. Whereas for the CHx in PU, they are all brought about from the monomers (DETA and IPDI), and kept unchanged before and after the polymerization because they are not involved in the polymerization.
Based on these considerations and taking into account of the amounts of DETA and IPDI used in PU synthesis, one can, theoretically, calculate the ratio of NHx/CHx for a given PU (see ESI,† Part 4, for details of the calculation). This same ratio of NHx/CHx can be also estimated from the peak heights (areas) of the relevant absorption. These were done for the linear (prepared using IPDI and H2O) and the crosslinked PU (prepared with different DETA–IPDI). The results are given in Table 6. For simplicity and clarity, only the final results, including theoretical and experimental NHx/CHx (obtained by peak height and by peak area) are given (detailed data are available in ESI,† Part 4).
| DETA | NHx | Area 1a | Height 1c | Theoretical NHx/CHx |
|---|---|---|---|---|
| IPDI | NCO | Area 2b | Height 2d | |
| a Peak area of NHx at 3360 cm−1.b Peak area of CHx at 2907 cm−1 and 2953 cm−1.c Peak height of NHx at 3360 cm−1.d Peak height of CHx at 2953 cm−1. | ||||
| IPDI–H2O | 0 | 0.4893 | 0.4096 | 0.2500 |
| 2/3 | 1/1 | 0.9573 | 0.6641 | 0.3125 |
| 2/9 | 1/3 | 1.4634 | 0.8942 | 0.2750 |
| 2/15 | 1/5 | 1.3653 | 0.8545 | 0.2656 |
| 2/30 | 1/10 | 1.0252 | 0.7480 | 0.2581 |
One can see that the theoretical ratio in the PU synthesized with IPDI and H2O is 0.25, the lowest among all the theoretical values. As to the crosslinked PUs prepared with IPDI and DETA, based on the composition of IPDI and DETA and keeping in mind the possible transformation of their groups as discussed above, it is obvious that the ratio of NHx/CHx must be decreasing with increased IPDI in PU synthesis as seen from the data in Table 6 (the column at right).
It is known that, through FTIR, the most reliable estimation for the composition of a substance is by its peak area when a peak is well separated and symmetric. Because the peaks at 2953 cm−1 and 2907 cm−1 were seriously overlapped, estimations of NHx and CHx were first done from the heights of the assigned peaks, i.e. the peak at 3360 cm−1 for NHx and that at 2953 cm−1 for CHx. The ratios of the peak heights (Height 1/Height 2 in Table 6), assumingly representing that of NHx/CHx in PU, are listed in Table 6 (2nd right column).
At first look, it is noted that all the experimentally NHx/CHx ratios, calculated from peak heights in IR spectra, were remarkably higher than the theoretical ones. In theoretical estimation for NHx, it was assumed that all monomers (IPDI and DETA) were reacted, and that IPDI in excess reacted with water when more NCO than NH2 was initially charged, followed by the step polymerization of IPDI with the in situ formed primary amine (isophorone diamine). Since NCO is also reactive towards water and this is a competitive reaction with its reaction with DETA, and primary amine is the product of this competitive reaction. It is easy to conceive that a larger ratio of NHx/NCO than the theoretical one is therefore expected.
As to the variation of NHx/CHx, the data show that this ratio is actually decreasing with decreased DETA–IPDI for the runs with DETA–IPDI ratio from 2/9 to 2/30, in agreement with that for the theoretical ratios. However, this trend in NHx/CHx variation is not followed by the run done with DETA–IPDI ratio of 2/3 and that without DETA, where smaller NHx/CHx ratio was detected from the FTIR spectra. It is to remind that the polymerization here is a step-growth polymerization. The polymerization is supposed to go more thoroughly when the functional groups are at equal molar amount, as is the case with DETA–IPDI ratio of 2/3 (NHx/NCO = 1), leaving therefore very little amount of amine groups in the final PU, leading to a smaller ratio for NHx/CHx by consequence. For the linear PU prepared without DETA, the cause for the observed low ratio for NHx/CHx is different from the run with equal amount of amine and isocyanate. First, based on the composition of IPDI, this ratio of NHx/CHx was expected to be lower than that when DETA was present; secondary, PU was produced by step-polymerization of IPDI with the in situ formed amine, which is commonly known to be much more active towards IPDI than water. This is to say that the in situ formed amine was consumed immediately upon their formation. Only the amine formed at the end of the polymerization, where IPDI is no more available to react with, remained in the system.
Finally, these experimental NHx/CHx ratios were also estimated through calculation of peak areas for the relevant peaks instead of peak heights. Since the chosen peaks, particularly those of CHx from 2800 cm−1 to 2960 cm−1, comprehends a group of small peaks, a simulated peak separation using a software (Origin 8.1) was used prior to the integration of peak areas. The results are also included in Table 6 (middle column) for reference. In comparison to the theoretical value (NHx/CHx), these data are more biased than those obtained from peak height. This may be an indication that the estimated results on PU composition from peak heights are more reliable than those from peak areas when the peaks are overlapped.
Conclusions
Precipitation polymerization of IPDI and DETA in binary solvent of water–acetone was carried out in order to prepare uniformed microspheres of crosslinked PU with controlled crosslinking. Influences of polymerization temperature, solvent composition, shaking rate and the amount of DETA on the microspheres were studied. It was found that highly uniform microspheres were obtained when the polymerization was effected between 30 °C and 50 °C while H2O–acetone ratio was kept at 30/70 for the solvent and with monomer concentration of 2 wt%. A slight decrease in the size of microspheres along with a slightly larger size distribution was observed with increased polymerization temperature. With increased water amount in the solvent, the polymerization rate was promoted, the size of microspheres regularly reduced and the yield enhanced though slightly. Microspheres with the best uniformity were observed with water content from 30 wt% to 40 wt%. Impact of shaking rate was minimal on microsphere size and size distribution but a slight increase in the yield of microspheres was detected with increased shaking rate. As to PU crosslinking in the microspheres, it was found that DETA amount was the most effective means, although the crosslinking was changed with limited extent with changes in solvent composition and polymerization temperature. The effective crosslinking in microspheres was also checked by tests on microsphere swellability and the light transmittance of their dispersion in acetic acid. Based purely on analysis on FTIR spectra of PU synthesized with different DETA amount, it was demonstrated that PU structure and its evolution can be well depicted and the results are in good agreement with those obtained by theoretical estimation. This work provides therefore a practical pathway to preparation of uniform PU microspheres with adjustable crosslinking, and it demonstrates also that FTIR is a reliable and powerful tool for estimation of the composition and structure of polyurea.Acknowledgements
This work is financial supported by Natural Science Foundation of China (NSFC, Grant #21274054 and #21304038).Notes and references
- J. Ge, H. Lee, L. He, J. Kim, Z. Lu, H. Kim, J. Goebl, S. Kwon and Y. Yin, J. Am. Chem. Soc., 2009, 131, 15687–15694 CrossRef CAS PubMed.
- Z. Cao, X. Zhang, Z. Zhao and L. Li, in Proceedings of SPIE, ed. Q. Xin and R. E. Parks, SPIE, Bellingham WA, 1998, vol. 3557, pp. 51–54 Search PubMed.
- T. Hayashi and Y. Ikada, Biotechnol. Bioeng., 1990, 35, 518–524 CrossRef CAS PubMed.
- X. Feng, Z. Gu, Y. Jin and Z. Su, Biotechnol. Tech., 1998, 12, 289–293 CrossRef CAS.
- R. C. Mehta, B. C. Thanoo and P. P. Deluca, J. Controlled Release, 1996, 41, 249–257 CrossRef CAS.
- P. Gupta and S. Parasher, World J. Pharm. Pharm. Sci., 2013, 2, 729–741 CAS.
- D. Mestach, Prog. Colloid Polym. Sci., 2004, 124, 37–41 CAS.
- R. S. Frank, J. S. Downey and H. D. H. Stöver, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2223–2227 CrossRef CAS.
- J. S. Downey, R. S. Frank, W. H. Li and H. D. H. Stöver, Macromolecules, 1999, 32, 2838–2844 CrossRef CAS.
- H. Jiang, H. Chen, G. Zong, X. Liu, Y. Liang and Z. Tan, Polym. Adv. Technol., 2011, 22, 2096–2103 CrossRef CAS.
- S. E. Shim, S. Yang, Y. H. Chang and S. J. Choe, Colloid Polym. Sci., 2004, 283, 41–48 CAS.
- X. Z. Kong, X. L. Gu, X. Zhu and L. Zhang, Macromol. Rapid Commun., 2009, 30, 909–914 CrossRef CAS PubMed.
- X. Jiang, X. Z. Kong and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4492–4497 CrossRef CAS.
- X. Jiang, X. Zhu and X. Z. Kong, Chem. Eng. J., 2012, 213, 214–217 CrossRef CAS PubMed.
- M. Lin, Z. Dong, B. Peng, M. Li and Z. Wu, Acta Polym. Sin., 2011, 1, 48–54 CrossRef.
- X. Z. Kong, W. Jiang, X. Jiang and X. Zhu, Polym. Chem., 2013, 4, 5776–5784 RSC.
- R. M. Fitch, Br. Polym. J., 1973, 5, 467–483 CrossRef CAS.
- C. S. Chern, Prog. Polym. Sci., 2006, 31, 443–486 CrossRef CAS PubMed.
- S. G. Entelis and O. V. Nesterov, Russ. Chem. Rev., 1966, 35, 917–930 CrossRef PubMed.
- W. Jiang, L. Zhang, X. L. Gu, X. Zhu and X. Z. Kong, Acta Polym. Sin., 2013, 4, 406–413 Search PubMed.
- S. Li and Y. Liu, Polyurethane Resin and Its Application, Chemical Industry Press, Beijing, 2002, p. 19 Search PubMed.
- N. Luo, D. N. Wang and S. K. Ying, Polymer, 1996, 37, 3577–3583 CrossRef.
- N. Luo, D. N. Wang and S. K. Ying, China Synth. Rubber Ind., 1997, 20, 25–28 CAS.
- D. G. Czechowicz, E. R. Castillo and A. Nikroo, Fusion Sci. Technol., 2002, 41, 190–192 Search PubMed.
- I. P. Lisovsky, V. G. Lytovchenko, D. O. Mazunov and A. Szekeres, Ukr. J. Phys., 2005, 50, 78–83 CAS.
- J. Kong and S. Yu, Acta Biochim. Biophys. Sin., 2007, 39, 549–559 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM pictures of the PU microspheres prepared by copolymerization of IPDI and DETA in H2O–acetone with H2O–acetone at 30/70 and 2 wt% of monomer concentration with varied reciprocating shaking rates; schematic presentation of copolymerization of IPDI with DETA; thermal properties of PU microspheres by TGA test; and demonstration for theoretical calculation of NHx/CHx in a polyurea and their estimation from FTIR spectra. See DOI: 10.1039/c4ra04206a |
| This journal is © The Royal Society of Chemistry 2014 |
