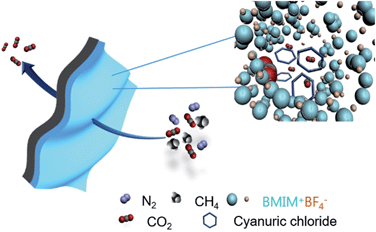
Facilitated CO2 transport and barrier effect through ionic liquid modified with cyanuric chloride†
Gil Hwan Hong‡
,
Dahye Ji‡ and
Sang Wook Kang*
Department of Chemistry, Sangmyung University, Seoul 110-743, Republic of Korea. E-mail: swkang@smu.ac.kr
First published on 26th March 2014
Abstract
A 1-butyl-3-methyl imidazolium tetrafluoroborate (BMIM+BF4−) ionic liquid membrane containing cyanuric chloride as a carrier can be utilized for facilitated CO2 transport. The cyanuric chloride complex was characterized by Fourier transform (FT) infrared spectroscopy, thermogravimetric analysis, viscosity measurements, and FT-Raman spectroscopy. The BMIM+BF4−/cyanuric chloride membrane showed a high CO2 permeance of 19.2 GPU, in addition to excellent ideal selectivities of 11.0 and 10.7 for CO2/N2 and 10.7 for CO2/CH4 mixtures, respectively. A barrier effect was observed for CH4 and N2, while the amine groups on the imidazolium ring and cyanuric chloride selectively interacted with CO2 molecules, thus facilitating their transport.
Global warming due to greenhouse gas (such as CO2) emission continues to be one of the major environmental problems.1,2 Carbon dioxide separation has been of specific interest to the research community, and many separation processes have been developed, including cryogenic separation, adsorption, and absorption.3 Compared to the traditional methods, membrane separation technology has various merits such as high energy efficiency, ease of operation, and environment friendliness.4,5 However, achieving high selectivity as well as high permeability remains one of the challenges to be overcome in the development of suitable membranes.6 As a solution, facilitated transport membranes (membranes containing carriers7) have been introduced. The carrier utilized in facilitated transport forms a reversible complex with the specific gas (CO2 or olefin gas), thereby increasing both selectivity and permeability.8
The imidazolium ring in ionic liquids (ILs) contains amine groups, which are known to be CO2 carriers.9–13 In addition, imidazolium-containing ILs have unique properties such as nonvolatility, high thermal stability, high ionic conductivity, and tunable solvation ability.14–17 For these reasons, imidazolium-containing ILs have been used for facilitated CO2 transport membranes. We have previously reported the CO2 separation performance of membranes containing 1-butyl-3-methyl imidazolium tetrafluoroborate (BMIM+BF4−) and copper nanoparticles (CuNPs). Such membranes utilized both the amine functionality of the imidazolium ring and the interface of the dipole on the CuNP surface caused by the anion of imidazolium. This membrane demonstrated reasonable separation performance, with a CO2 permeance of 17 GPU (1 GPU = 1 × 10−6 cm3 (STP) (cm−2 s−1 cm−1 Hg−1)) and an ideal selectivity of 5.0 and 4.8 for CO2/CH4 and CO2/N2, respectively.18 We have also recently reported the CO2 separation performance of PVP/potassium fluoride (KF) membranes that utilized the interaction between K+ ions and CO2 molecules. The PVP/KF membrane performance showed a CO2 permeance of 28.0 GPU and an ideal selectivity of 4.1 for CO2/N2.19 However, conventional membranes utilizing metal nanoparticles generally showed low gas permeability, which remains one of the most important challenges for commercialization.7
Herein, we present a new strategy for developing facilitated transport membranes based on ILs in concert with cyanuric chloride. Cyanuric rings having amine groups are expected to interact reversibly with CO2, while other gas molecules such as CH4 and N2 can be blocked by the barrier effect of the cyclic ring (Scheme 1). The synergistic effect of both the amine group of the ionic liquid and the amine group of cyanuric chloride was investigated. The decomposition characteristics of the BMIM+BF4−/cyanuric chloride complexes were assessed by TGA and are shown in Fig. 1 Cyanuric chloride decomposed over the temperature range 50–110 °C, and BMIM+BF4− evaporated over the temperature range 315–470 °C. The TGA curve of the prepared BMIM+BF4−/cyanuric chloride mixture showed two shoulders at 90–190 °C (weight loss ∼9%) and 320–480 °C, indicating that the first dip was attributed to cyanuric chloride decomposition. The BMIM+BF4−/cyanuric chloride mixture decomposed slowly and at a higher temperature than did neat cyanuric chloride, indicating the interaction between cyanuric chloride and BMIM+BF4−.
For a detailed analysis of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band of cyanuric chloride complexed to BMIM+BF4−, the FT-IR spectrum was deconvoluted, as shown in Fig. 2 The C
N band of cyanuric chloride complexed to BMIM+BF4−, the FT-IR spectrum was deconvoluted, as shown in Fig. 2 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond attributable to cyanuric chloride had one peak at 1492 cm−1. When cyanuric chloride was incorporated into BMIM+BF4−, the C
N bond attributable to cyanuric chloride had one peak at 1492 cm−1. When cyanuric chloride was incorporated into BMIM+BF4−, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peak shifted to 1511 cm−1 due to the interaction of carbon atom to have positive polarity with anions of BMIM+BF4− in cyanuric chloride. Interactions between cyanuric chloride and BMIM+BF4− in the BMIM+BF4−/cyanuric chloride mixture were also investigated by FT-Raman spectroscopy.
N peak shifted to 1511 cm−1 due to the interaction of carbon atom to have positive polarity with anions of BMIM+BF4− in cyanuric chloride. Interactions between cyanuric chloride and BMIM+BF4− in the BMIM+BF4−/cyanuric chloride mixture were also investigated by FT-Raman spectroscopy.
 | ||
| Fig. 2 FT-IR spectra for BMIM+BF4−, cyanuric chloride, and BMIM+BF4−/cyanuric chloride mixture (1/0.1). | ||
The Raman spectra in the regions of the BF4− stretching bands are shown in Fig. 3. The peaks for free ions, ion pairs, and ion aggregates of BF4− appeared at 765, 770, and 774 cm−1, respectively.18 The peak of neat BMIM+BF4− was appeared predominately at 772.5 cm−1, indicating that the existence of ion aggregates and ion pairs. When cyanuric chloride was added, the peak shifted to 765 cm−1 because of the release of free BF4 from the BMIM+BF4−of IL. This result suggests the interaction of BMIM+BF4− with cyanuric chloride. A change in the viscosity of BMIM+BF4− was observed after cyanuric chloride was added (Table 1). When cyanuric chloride was incorporated into BMIM+BF4−, the viscosity increased to 129.5 cP, while that of neat BMIM+BF4− was 92.9 cP. These results suggest that cyanuric chloride was well dispersed in BMIM+BF4−.
 | ||
| Fig. 3 FT-Raman spectra in the BF4− stretching region for neat BMIM+BF4− and BMIM+BF4−/cyanuric chloride mixture. | ||
| Neat BMIM+BF4− | BMIM+BF4−/cyanuric chloride | |
|---|---|---|
| Viscosity (cP) | 92.9 | 129.5 |
Finally, the separation performance of the BMIM+BF4−/cyanuric chloride membrane was investigated. Values for CO2 selectivity and permeance were measured for CO2/N2 and CO2/CH4 mixtures (Table 2). The CO2, CH4, and N2 permeance of a pure BMIM+BF4− membrane were 17.0, 5.0, and 3.4 GPU, respectively.
| Permeance (GPU) | Selectivity | ||||
|---|---|---|---|---|---|
| N2 | CH4 | CO2 | CO2/N2 | CO2/CH4 | |
| Neat BMIM+BF4−18 | 3.4 | 5.0 | 17.0 | 5.0 | 4.8 |
| BMIM+BF4−/cyanuric chloride | 1.7 | 1.8 | 19.2 | 11.0 | 10.7 |
Thus, the ideal selectivities for the CO2/CH4 and CO2/N2 mixtures were 4.8 and 5.0, respectively, because CO2 interacts only with the amine groups of the imidazolium ring. When cyanuric chloride was incorporated into BMIM+BF4−, the permeances of N2, CH4, and CO2 were 1.7, 1.8, and 19.2 GPU, respectively. Thus, the ideal selectivities of the CO2/CH4 and CO2/N2 mixtures were 11.0 and 10.7, respectively. The ideal selectivity and CO2 permeance of the BMIM+BF4−/cyanuric chloride membrane were both higher than those of neat BMIM+BF4−. These improvements can be attributed to two factors: (1) the carrier effect of the amine in cyanuric chloride and (2) the barrier effect for N2 and CH4 caused by the cyanuric chloride ring. As a result, the BMIM+BF4−/cyanuric chloride membrane showed superior CO2 separation performance because of the combination of facilitated CO2 transport and the barrier effect as shown in Scheme 2.
Conclusions
A newly designed membrane based on both facilitated CO2 transport and a barrier effect was demonstrated using cyanuric chloride in concert with ionic liquids. The BMIM+BF4−/cyanuric chloride membrane showed an improvement in the ideal selectivity for CO2/CH4 and CO2/N2 mixtures (with an observed CO2 permeance of 19.2 GPU) over neat BMIM+BF4−. The improved separation performance of up to 170% was attributed to both facilitated transport and the barrier effect. The amine functionalities of cyanuric chloride and BMIM+BF4− both interact with CO2 to facilitate transport, while the cyclic ring of cyanuric chloride can act as a barrier for N2 and CH4 molecules.Acknowledgements
This work was supported by an Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean government Ministry of Trade, Industry and Energy (20122010100040). This work was also supported by the Basic Science Research Program (2013021962) and Korea CCS R&D Center through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.Notes and references
- A. L. Kohl and R. B. Nielsen, Gas Purification, Gulf Publishing, Houston, TX, 5th edn, 1997 Search PubMed.
- A. L. Khan, X. Li and I. F. J. Vankelecom, J. Membr. Sci., 2011, 372, 87 CrossRef CAS PubMed.
- O. Davidson and B. Metz, Special Report on Carbon Dioxide Capture and Storage, International Panel on Climate Change, 2005 Search PubMed.
- W. Yave, A. Car, J. Wind and K. V. Peinemann, Nanotechnology, 2010, 21, 395301 CrossRef PubMed.
- W. J. Koros and G. K. Fleming, J. Membr. Sci., 1993, 83, 1 CrossRef CAS.
- L. Robeson, J. Membr. Sci., 2008, 320, 390 CrossRef CAS PubMed.
- L. Deng, T. J. Kim and M. B. Hägg, J. Membr. Sci., 2009, 340, 154 CrossRef CAS PubMed.
- L. A. El-Azzami and E. A. Grulke, J. Membr. Sci., 2009, 328, 15 CrossRef CAS PubMed.
- K. E. Gutowski and E. J. Maginn, J. Am. Chem. Soc., 2008, 130, 14690 CrossRef CAS PubMed.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926 CrossRef CAS PubMed.
- B. E. Gurkan, J. C. de la Fuente, E. M. Mindrup, L. E. Ficke, B. F. Goodrich, E. A. Price, W. F. Schneider and J. F. Brennecke, J. Am. Chem. Soc., 2010, 132, 2116 CrossRef CAS PubMed.
- C. Wang, H. Luo, D. Jiang, H. Li and S. Dai, Angew. Chem., Int. Ed., 2010, 49, 5978 CrossRef CAS PubMed.
- C. Wang, X. Luo, H. Luo, D. Jiang, H. Li and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 4978 Search PubMed.
- D. D. Iarikov, P. Hacarlioglu and S. T. Oyama, Chem. Eng. J., 2011, 166, 401 CrossRef CAS PubMed.
- J. E. Bara, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2008, 47, 9919 CrossRef CAS.
- D. Camper, J. E. Bara, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2008, 47, 8496 CrossRef CAS.
- P. Scovazzo, D. Havard, M. McShea, S. Mixon and D. Morgan, J. Membr. Sci., 2009, 327, 41 CrossRef CAS PubMed.
- J. H. Lee, J. Hong, J. H. Kim, Y. S. Kang and S. W. Kang, Chem. Commun., 2012, 48, 5298 RSC.
- J. H. Oh, Y. S. Kang and S. W. Kang, Chem. Commun., 2013, 49, 10181 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental method, characterisation and material. See DOI: 10.1039/c4ra00945b |
| ‡ These authors contributed equally to this work as first authors. |
| This journal is © The Royal Society of Chemistry 2014 |