Silymarin coated gold nanoparticles ameliorates CCl4-induced hepatic injury and cirrhosis through down regulation of hepatic stellate cells and attenuation of Kupffer cells†
Nurul
Kabir
a,
Hamid
Ali
a,
Muhammad
Ateeq
b,
Massimo F.
Bertino
c,
Muhammad Raza
Shah
*b and
Louis
Franzel
c
aDr Panjwani Center for Molecular Medicine and Drug Research, University of Karachi, Karachi, Pakistan
bH.E.J Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
cDepartment of Physics, Virginia Commonwealth University, Richmond, VA, USA. E-mail: raza.shah@iccs.edu
First published on 21st January 2014
Abstract
Silymarin coated gold nanoparticles with a mean size of 20 nm were synthesized and functionalized in one pot using silymarin as a reducing and stabilizing agent. Conjugation of gold with silymarin was confirmed with FT-IR and UV-visible techniques. The aim of this study was to investigate the hepatoprotective and antifibrotic potential of Silymarin coated gold nanoparticles. For this purpose oxidative liver damage was induced in Wistar rats by intraperitoneal injection of CCl4 dissolved in olive oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 1 ml kg−1). Silymarin coated gold nanoparticles were administered intragastrically once per day for 14 weeks in a dose of 30 mg kg−1 of body weight. Hepatoprotective and antifibrotic activities of silymarin coated gold nanoparticles were assessed in terms of reduction in serum enzymes (ALT, AST, ALP), through histopathology and immunohistochemistry techniques. It also reduced the CCl4-induced damaged area as well as fibrotic area to 0% as assessed by histopathology. The Alpha SMA and Kupffer cells were also reduced in number around the portal traid area by the silymarin coated gold nanoparticles. These hepatoprotective and antifibrotic effects were better than the positive control silymarin. Our results suggest the therapeutic effect of silymarin coated gold nanoparticles in CCl4-induced liver injury and cirrhosis by promoting extracellular matrix degradation, hepatic stellate cells inactivation with strong enhancement of hepatic regenerative capacity. Silymarin coated gold nanoparticles could be administered for up to 14 weeks without inducing side effects or alterations of the histological structure of kidneys, heart, pancreas and lungs.
1 v/v, 1 ml kg−1). Silymarin coated gold nanoparticles were administered intragastrically once per day for 14 weeks in a dose of 30 mg kg−1 of body weight. Hepatoprotective and antifibrotic activities of silymarin coated gold nanoparticles were assessed in terms of reduction in serum enzymes (ALT, AST, ALP), through histopathology and immunohistochemistry techniques. It also reduced the CCl4-induced damaged area as well as fibrotic area to 0% as assessed by histopathology. The Alpha SMA and Kupffer cells were also reduced in number around the portal traid area by the silymarin coated gold nanoparticles. These hepatoprotective and antifibrotic effects were better than the positive control silymarin. Our results suggest the therapeutic effect of silymarin coated gold nanoparticles in CCl4-induced liver injury and cirrhosis by promoting extracellular matrix degradation, hepatic stellate cells inactivation with strong enhancement of hepatic regenerative capacity. Silymarin coated gold nanoparticles could be administered for up to 14 weeks without inducing side effects or alterations of the histological structure of kidneys, heart, pancreas and lungs.
1. Introduction
Metal and oxide nanoparticles are currently being considered for a wide array of medical applications, including sensing,1–5 photodynamic therapy,6 drug delivery,7 imaging8 and hyperthermia.9 In most cases, chemically inert nanoparticles are employed to minimize toxicity and side effects. For example, Au nanoparticles are often employed for drug delivery10 and photothermal therapy,11 and Fe oxide nanoparticles are employed for MRI12 and hyperthermia.13 Chemically reactive materials are seldom employed, typically only as biocides for external application. For example, Ag nanoparticles have been employed for millennia as antibacterials and can now be found in commercial detergents14 and in clothing.15 Cu nanoparticles, another well-known biocide, are being employed as antifungals and antibacterials for clothing.16 Several reports have also shown17–21 that the antibacterial activity of several biocides can be amplified by conjugation to Ag nanoparticles. These results are not completely surprising, since the conjugates couple two biocides, one organic (the capping agents) and one inorganic (the Ag nanoparticles). These two biocides can affect different metabolic pathways of the targeted organisms and can thus be more effective than each component taken individually. Recent reports, however, have suggested that nanoparticles of chemically inert materials can also enhance the potency of certain molecules. For example, the group of Kotov showed that leukemia cells were killed more efficiently by conjugation of 6-mercaptopurine to Au nanoparticles.22 Jin and He23 showed that the antibacterial activity of nisin increased when MgO was added to a culture of food borne pathogens such as E. coli, and our own group24 showed that conjugation to Au nanoparticles increased by up to three times the antibacterial, antifungal, insecticidal and cytotoxic activity of the biocide 2–4, dihydroxybenzene carbodithioic acid (DHT). The latter results, they showed that conjugation to Au nanoparticles increased the activity of drugs not only against unicellular organisms but also against more complex organisms such as brine shrimp larvae and insects such as fruit parasites. We therefore wondered if the enhanced activity of Au bioconjugates could be a more general phenomenon, and, specifically, if Au nanoparticle conjugates could improve the therapeutic activity of drugs used to treat complex pathologies. As a model system, we focused on oxidative liver damage. Liver is a convenient target organ since it removes bioconjugates very efficiently from the blood stream.25 By choosing a liver pathology, the conjugates do not need to be protected from liver absorption, which simplifies synthesis and data interpretation. In addition, oxidative liver damage, which ultimately leads to cirrhosis, is easy to induce in animals and it is also a clinically relevant and widespread pathology. Liver cirrhosis is a relatively common disease which in the industrialized world is often associated to excessive alcohol consumption. Cirrhosis, however, can be also caused by the Hepatitis B and C viruses, which are estimated to affect 500 million people worldwide and are especially widespread in developing countries. In Pakistan alone, with a population of about 150 million, data from the last 10 years show a prevalence of 3–4% for hepatitis B26 and 5–7% for hepatitis C.27Here, we show in vivo that conjugation to Au nanoparticles enhances the therapeutic effect of silymarin, a flavonoid that is being considered for the treatment of several hepatic pathologies. Au–silymarin conjugates induced complete recovery from oxidative liver damage and protected from cirrhosis. Administration of silymarin, our positive control, leads to a partial recovery of hepatic structure and enzymatic levels, while Au nanoparticles had no therapeutic effect. Most importantly, Au–silymarin conjugates could be administered for up to 14 weeks without any noticeable side effects and without inducing any discernible changes in the histological structure of heart, kidney and lungs of the animals.
2. Results and discussion
2.A. Nanoparticle synthesis
Silymarin is a mild reducing agent, and during addition the color of the reaction mixture changed from yellow to violet, indicating reduction of the Au ions and formation of Au nanoparticles. UV-vis spectra (Fig. S1†) of the suspensions revealed an absorption maximum at 520 nm, which is within the range expected for non-coagulated suspensions of Au nanoparticles.28 Conjugation of silymarin to the gold nanoparticles was supported by FTIR spectroscopy,38 reported in Fig. S.2.† Transmission electron microscopy (TEM) showed that the nanoparticles had a narrow size distribution with a mean size of 20 nm, as shown in Fig. S3.†2.B. Biological studies
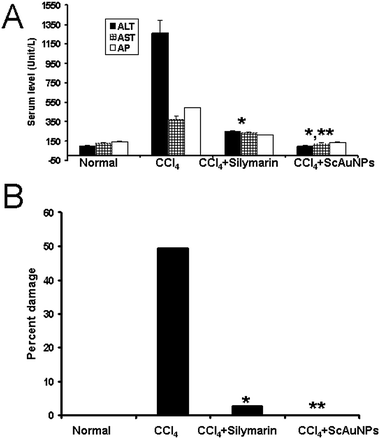
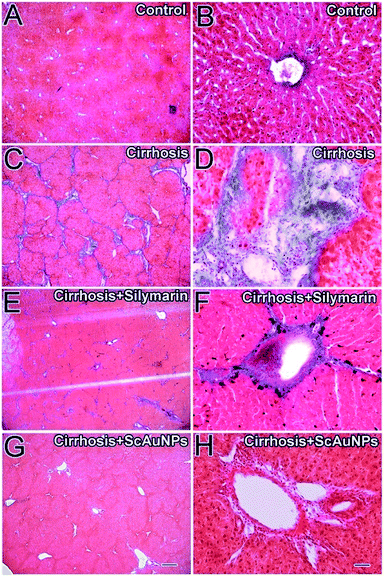
Although viral hepatitis is the most common cause of liver injury and inflammation, in this study we used the widely accepted CCl4-induced model because the rats are naturally immune to the hepatitis B and C virus. Carbon tetrachloride (CCl4)-induced liver injury is a widely used and well-characterized animal model of xenobiotic-induced, oxidative stress-mediated hepatotoxicity.29 CCl4 induces the production of several types of reactive oxygen species (ROS) via cytochrome P450. The ensuing liver injury presents symptoms which are similar to those of chronic liver injury in humans.30,31Masson's trichrome stain was used for the detection of fibrosis of liver which is easily identified by a blue coloration. The livers from control rat stained with Masson's trichrome stain showed traces of fibrosis only in the vascular walls (Fig. 4A and B). Liver section from the cirrhosis model group showed extensive multiple nodular fibrosis predominantly in the portal triad areas (Fig. 4C and D). Some fibrotic lesions were still present in the livers of rats treated with 500 mg kg−1 per day of standard silymarin for 14 weeks (Fig. 4E and F), but the fibrosis was reduced and less frequent compared to the cirrhosis model group, whereas pericellular fibrosis was observed in the surrounding liver parenchyma (Fig. 4E and F). The livers of rat treated with ScAuNPs at a dose of 30 mg kg−1 per day for 14 weeks showed only minor, markedly small fibrotic deposits in the periportal areas (Fig. 4G and H). The hepatocytes were looking undamaged with distinct hepatocyte cell morphology and sinusoidal spaces (Fig. 4H). However, both the enzyme level (Fig. 5A) and the calculation of fibrotic area (Fig. 5B) indicated liver damage with ∼3.5% fibrosis with some mixed inflammatory infiltrate around the portal tracts area in the silymarin treated group. In contrast, the liver of rats receiving 30 mg kg−1 of (ScAuNPs) showed normal hepatic architecture with almost no sign of hepatic fibrosis or inflammation (Fig. 4G and H and 5A and B). Both the enzyme level (**, P < 0.001, Fig. 5A) and histopathological analysis of fibrosis (**, P < 0.001, Fig. 5B) showed that this protective effect was more than the positive control silymarin.
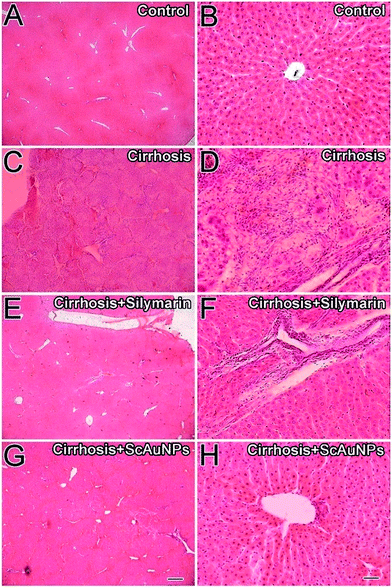
To further confirm the enhanced protective action of the Au nanoparticle conjugates, a detailed immunohistochemical characterization of the rats treated for 14 weeks was carried out. Hepatic stellate cells (HSCs) were identified using the α-SMA antibody. In the livers of control animals the distribution of α-SMA-positive fibrogenic cells was restricted to the smooth musculature and to the portal and central veins, while other liver areas remained negative (Fig. 6A–D). Immunohistochemical detection of α-SMA as a marker for hepatic stellate cells (HSCs) activation revealed minimal staining in the blood vessels (Fig. 6A–D). CCl4-induced liver cirrhosis strongly induced perisinusoidal α-SMA expression, which are activated HSCs (Fig. 6E). α-SMA positive cells were located at the scar–parenchyma interface (Fig. 6H). The HSCs were mostly present in the periportal and in the central lobular area along the sinusoidal walls (Fig. 6E and H). Rats treated with Silymarin as a positive control showed marked positive hepatic stellate cells along the side of sinusoidal spaces and in the portal triad areas (Fig. 6I and L). Rats treated with ScAuNPs showed complete reversion of α-SMA positive HSCs (Fig. 6M). The livers of these rats showed a staining pattern similar to the control group's α-SMA positive cells in the vicinity of portal triad (Fig. 6P and D). The liver macrophage cells (Kupffer cells, KCs) were identified using the CD68 antibody. The CD68+ KCs, in the livers of control animals, were present in hepatic sinusoids and were at low number (Fig. 7A and D). Immunohistochemical detection of CD68+ KCs revealed minimal staining in the sinusoid of the normal control group (Fig. 7D). In liver cirrhosis model group, CD68+ KCs with strong staining appeared not only in hepatic sinusoids, but also in portal areas and adjacent to fibrotic septa (Fig. 7E and H). The rats of the positive control group showed marked positive KCs along the side of sinusoidal spaces as well as in the portal triad areas (Fig. 7I and L). Rats treated with ScAuNPs showed CD68+ KCs picture that was very similar to the control (Fig. 7M).
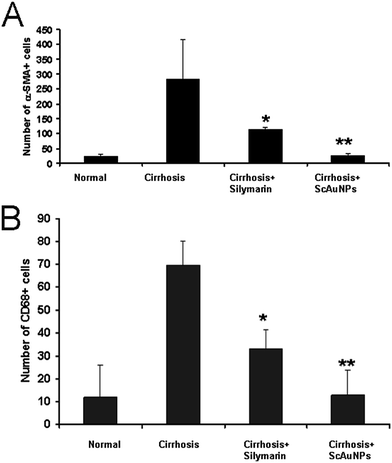
The number of α-SMA+ cells in the portal triad area is reported in Fig. 8A. A decreased (yet elevated from the control group) number of α-SMA+ cells were noticed in the positive control group compared to the cirrhosis model group. In contrast, the liver of rats treated with ScAuNPs showed a number of α-SMA+ cells similar to the control group. The number of CD68+ KCs in the portal triad area is reported in Fig. 8B. CD68+ KCs revealed an increase in their number in the cirrhosis group compared to the control. In the positive control group the number of CD68+ KCs decreased compared to the cirrhosis group but were still higher than the control group. In contrast, the liver of rats treated with ScAuNPs showed a number of CD68+ KCs which was lower than the positive control and quite comparable to the positive control group.
Most important, treatment with ScAuNPs showed no toxicity in a 14 weeks study. No symptoms of toxicity such as weight loss, piloerection, immobility etc. were observed. A post-mortem study was performed which showed no sign of damage to liver, kidney, heart, pancreas, stomach, intestine, lungs spleen etc. Histopathological analysis was performed of liver, kidney, heart, and pancreas tissue which all showed a normal histological picture (Fig. 9).
Thus, our results show that ScAuNPs have a superior hepatoprotective activity than pure silymarin. Now, one of the main issues of silymarin is its poor solubility, which leads to a bioavailability of between 20 and 50% of a dose administered orally.35 The solubility of ScAuNPs, on the other hand, is very high. Our conjugates could be dissolved in water at concentrations as high as 200 mg ml−1. For this reason, rats were fed with different amounts of drugs. The positive control group was given 200 mg kg−1 of pure silymarin in the acute toxicity experiments and 500 mg kg−1 in the chronic liver injury experiments. This corresponded to an effective dose of 40 mg kg−1 for the worst-case scenario of 20% bioavailability (100 mg kg−1 for the chronic study) and of 100 mg kg−1 in the best-case scenario of 50% bioavailability (250 mg kg−1 for the chronic study). The group treated with ScAuNPs was given a dose of 30 mg kg−1 in all studies. Given the high solubility of ScAuNPs, we can assume that the full amount will become bioavailable. However, one must remember that ScAuNPs are, for the most part, Au, and that silymarin represents only a minor weight fraction of the conjugates. In recent experiments,24 for example, we determined that carbothiamate surfactants represented only about 7% of the weight of 20 nm diameter Au nanoparticle conjugates. Kotov et al.22 synthesized Au-mercaptopurine conjugates with a nanoparticle diameter of about 4 nm and a 50% weight of organics. The %weight of the organics scales with the surface-to-volume ratio, which in turn depends on the radius of the nanoparticles. For our ∼10 nm nanoparticles we therefore estimate a silymarin content between 15 and 20 wt%. Thus, the groups treated with ScAuNPs received an effective silymarin dose between 4.5 and 6 mg kg−1, which is about an order of magnitude lower than the silymarin received by the positive control group. We conclude that the superior protective effect of ScAuNPs is not due to solubility but, rather, to conjugation. Our conclusion is corroborated by recent work by Parveen et al.36,37 In this work, silymarin solubility and bioavailability were increased 4 to 6 times by a nanoemulsification technique. Liver damage was induced chemically in rats, and nanoemulsions were found to improve the protective action of silymarin. However, the increased solubility did not lead to complete recovery of the hepatic functions. Histological analysis showed that the liver of rats treated with nanoemulsified silymarin exhibited an abnormal number of fat vacuoles. Enzymatic levels were also improved when compared to the untreated group, but they were higher than the control group and comparable to the levels of the positive control group. In our experiments, instead, histology and enzymatic levels of the group treated with ScAuNPs were undistinguishable from the control group of healthy animals.
3. Conclusion
In conclusion, we have shown that conjugates of silymarin and Au nanoparticles are more effective than the positive control silymarin in preventing and repairing acute and chronic liver damage. Animals treated with the Au conjugates showed a normal liver structure and hepatic functions. In contrast, animals treated with the positive control silymarin showed residual damage and inflammation and a slightly abnormal liver function. The nanoparticles conjugates did not show any toxic effects or alterations of the liver enzymatic levels even after 14 weeks of treatment.4. Experimental details
4.A. Materials synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of AuNPs (after centrifugation) and KBr. Transmission electron spectroscopy (TEM) was carried out with a Zeiss Libra operated at 120 keV.
1 mixture of AuNPs (after centrifugation) and KBr. Transmission electron spectroscopy (TEM) was carried out with a Zeiss Libra operated at 120 keV.
4.B. Biology experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. Group III was treated with the standard drug silymarin (200 mg/kg body weight, p.o.) daily for 3 days, and also received CCl4–olive oil (1
1 v/v. Group III was treated with the standard drug silymarin (200 mg/kg body weight, p.o.) daily for 3 days, and also received CCl4–olive oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 1 ml kg−1 body weight, i.p.) on 3rd day 1 hour after administration of silymarin. Groups IV (treated group) were administered orally a dose of 30 mg kg−1 body weight of gold nano-particles (p.o.), for 3 days in addition received CCl4–olive oil (1
1 v/v, 1 ml kg−1 body weight, i.p.) on 3rd day 1 hour after administration of silymarin. Groups IV (treated group) were administered orally a dose of 30 mg kg−1 body weight of gold nano-particles (p.o.), for 3 days in addition received CCl4–olive oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 1 ml kg−1 body weight, i.p.) on 3rd day 1 hour after intraperitoneal injection of CCl4. After 48 hours of treatment the animals were sacrificed, blood was collected and allowed to clot, and serum was separated for assessment of biochemical parameters.
1 v/v, 1 ml kg−1 body weight, i.p.) on 3rd day 1 hour after intraperitoneal injection of CCl4. After 48 hours of treatment the animals were sacrificed, blood was collected and allowed to clot, and serum was separated for assessment of biochemical parameters.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, i.p. twice a week for 14 weeks. Group III (Silymarin treated group) was treated for 14 weeks with the standard drug silymarin (500 mg/kg per day body weight, i.g.) after 3 days of CCl4–olive oil (1
1 v/v, i.p. twice a week for 14 weeks. Group III (Silymarin treated group) was treated for 14 weeks with the standard drug silymarin (500 mg/kg per day body weight, i.g.) after 3 days of CCl4–olive oil (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 1 ml kg−1 body weight, i.p) single injection. Group IV (silymarin–Au nanoparticle conjugates treated group) received a single intraperitoneal injection of carbon tetrachloride (1
1 v/v, 1 ml kg−1 body weight, i.p) single injection. Group IV (silymarin–Au nanoparticle conjugates treated group) received a single intraperitoneal injection of carbon tetrachloride (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 1 ml kg−1 body weight, i.p. twice a week) before 3 days followed by daily treatment with silymarin–Au nanoparticles at a dose of (30 mg kg−1 per day, i.g) for 14 weeks. After 3 days of the final injection of CCl4, rats were anesthetized with sodium pentothal. Animals were starved overnight before sacrifice. The serum was stored at −26 °C after separation until assayed. The liver and kidney were carefully dissected and cleaned of extraneous tissue, and part of the liver tissue was immediately transferred into 10% formalin for histopathological investigation.
1 v/v, 1 ml kg−1 body weight, i.p. twice a week) before 3 days followed by daily treatment with silymarin–Au nanoparticles at a dose of (30 mg kg−1 per day, i.g) for 14 weeks. After 3 days of the final injection of CCl4, rats were anesthetized with sodium pentothal. Animals were starved overnight before sacrifice. The serum was stored at −26 °C after separation until assayed. The liver and kidney were carefully dissected and cleaned of extraneous tissue, and part of the liver tissue was immediately transferred into 10% formalin for histopathological investigation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) for 1 hour at 37 °C. For hepatic stellate cells (HSCs) monoclonal α-SMA antibody (clone 1A4, Sigma) and for liver macrophages monoclonal CD68 (clone ED1, abcam) antibody was used. After thoroughly washing with PBS, the sections were incubated with a mixture of Texas Red-conjugated goat anti-mouse IgG (1
100) for 1 hour at 37 °C. For hepatic stellate cells (HSCs) monoclonal α-SMA antibody (clone 1A4, Sigma) and for liver macrophages monoclonal CD68 (clone ED1, abcam) antibody was used. After thoroughly washing with PBS, the sections were incubated with a mixture of Texas Red-conjugated goat anti-mouse IgG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) for 40 min. After washing extensively with PBS the nuclei were stained with DAPI (1
100) for 40 min. After washing extensively with PBS the nuclei were stained with DAPI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fold dilution of a 1 mg ml−1 stock solution) for 1 min, washed with PBS and mounted in mounting media. The fluorescent images were acquired and analyzed using a Nikon 90i multichannel fluorescence microscope and Nikon DXM 1200C camera with NIS-Elements image analysis software AR 3.0 (Nikon). Finally, image processing was performed with Adobe Photoshop software.
000 fold dilution of a 1 mg ml−1 stock solution) for 1 min, washed with PBS and mounted in mounting media. The fluorescent images were acquired and analyzed using a Nikon 90i multichannel fluorescence microscope and Nikon DXM 1200C camera with NIS-Elements image analysis software AR 3.0 (Nikon). Finally, image processing was performed with Adobe Photoshop software.
References
- J. H. W. Leuvering, P. J. H. M. Thal, D. D. White and A. H. W. M. Schuurs, J. Immunol. Methods, 1983, 62, 163–174 CrossRef.
- T. Gribnau, J. Leuvering and H. Van Hell, J. Chromatogr. B: Biomed. Sci. Appl., 1986, 376, 175–189 CrossRef.
- P. Englebienne, A. V. Hoonacker and M. Verhas, J. Spectrosc., 2003, 17, 255–273 CrossRef PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef.
- V. Bogatyrev, L. Dykman, Y. M. Krasnov, V. Plotnikov and N. Khlebtsov, Colloid J., 2002, 64, 671–680 CrossRef.
- F. Lu, T. L. Doane, J.-J. Zhu and C. Burda, Inorg. Chim. Acta, 2012 DOI:10.1016/j.ica.2012.05.038.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef PubMed.
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238–7248 CrossRef PubMed.
- P. Diagaradjane, A. Shetty, J. C. Wang, A. M. Elliott, J. Schwartz, S. Shentu, H. C. Park, A. Deorukhkar, R. J. Stafford and S. H. Cho, Nano Lett., 2008, 8, 1492–1500 CrossRef PubMed.
- G. Han, P. Ghosh and V. M. Rotello, Nanomedicine, 2007, 2, 113–123 CrossRef PubMed.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers Med. Sci., 2008, 23, 217–228 CrossRef PubMed.
- B. Chertok, B. A. Moffat, A. E. David, F. Yu, C. Bergemann, B. D. Ross and V. C. Yang, Biomaterials, 2008, 29, 487–496 CrossRef PubMed.
- F. Sonvico, S. Mornet, S. Vasseur, C. Dubernet, D. Jaillard, J. Degrouard, J. Hoebeke, E. Duguet, P. Colombo and P. Couvreur, Bioconjugate Chem., 2005, 16, 1181–1188 CrossRef PubMed.
- C. A. Impellitteri, T. M. Tolaymat and K. G. Scheckel, J. Environ. Qual., 2009, 38, 1528–1530 CrossRef PubMed.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef.
- http://www.cupron.com/ .
- A. Panáček, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. j. Nevečná and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef PubMed.
- C. Baker, A. Pradhan, L. Pakstis, D. J. Pochan and S. I. Shah, J. Nanosci. Nanotechnol., 2005, 5, 244–249 CrossRef PubMed.
- H. Kong and J. Jang, Langmuir, 2008, 24, 2051–2056 CrossRef PubMed.
- M. Singh, S. Singh, S. Prasad and I. Gambhir, Digest J. Nanomater. Biostructures, 2008, 3, 115–122 Search PubMed.
- X. Xu, Q. Yang, Y. Wang, H. Yu, X. Chen and X. Jing, Eur. Polym. J., 2006, 42, 2081–2087 CrossRef PubMed.
- P. Podsiadlo, V. A. Sinani, J. H. Bahng, N. W. S. Kam, J. Lee and N. A. Kotov, Langmuir, 2008, 24, 568–574 CrossRef PubMed.
- T. Jin and Y. He, J. Nanopart. Res., 2011, 13, 6877–6885 CrossRef.
- S. S. Naz, N. U. Islam, M. R. Shah, S. S. Alam, Z. Iqbal, M. Bertino, L. Franzel and A. Ahmed, J. Nanobiotechnol., 2013, 11, 13 CrossRef PubMed.
- S. Stolnik, L. Illum and S. Davis, Adv. Drug Delivery Rev., 1995, 16, 195–214 CrossRef.
- F. André, Vaccine, 2000, 18, S20–S22 CrossRef.
- S. J. Zuberi, International Hepatology Communications, 1996, 5, 19–26 CrossRef.
- U. Kreibig and M. Vollmer, Optical properties of metal clusters, Springer Verlag, 1995 Search PubMed.
- R. O. Rechnagel, E. A. Glende and G. L. Plaa, Crit. Rev. Toxicol., 1973, 2, 263–297 CrossRef PubMed.
- S. Basu, Toxicology, 2003, 189, 113–127 CrossRef.
- L. W. Weber, M. Boll and A. Stampfl, Crit. Rev. Toxicol., 2003, 33, 105–136 CrossRef PubMed.
- G. Poli, Mol. Aspects Med., 2000, 21, 49–98 CrossRef.
- H. Tsukamoto, M. Matsuoka and S. French, Experimental models of hepatic fibrosis: a review, 1990 Search PubMed.
- G. K. Marathe, K. A. Harrison, L. J. Roberts, J. D. Morrow, R. C. Murphy, L. W. Tjoelker, S. M. Prescott, G. A. Zimmerman and T. M. McIntyre, J. Lipid Res., 2001, 42, 587–596 Search PubMed.
- S. Javed, K. Kohli and M. Ali, Alternative Med. Rev., 2011, 16, 239–249 Search PubMed.
- R. Parveen, S. Baboota, J. Ali, A. Ahuja, S. S. Vasudev and S. Ahmad, Int. J. Pharm., 2011, 413, 245–253 CrossRef PubMed.
- R. Parveen, S. Baboota, J. Ali, A. Ahuja, S. S. Vasudev and S. Ahmad, Arch. Pharmacal Res., 2011, 34, 767–774 CrossRef PubMed.
- N. A. Begum, S. Mondal, S. Basu, R. A. Laskar and D. Mandal, Colloids Surf., B, 2009, 71, 113–118 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46093b |
| This journal is © The Royal Society of Chemistry 2014 |