Following initial changes in nanoparticle films under laminar flow conditions with in situ GISAXS microfluidics
Volker
Körstgens
a,
Martine
Philipp
a,
David
Magerl
a,
Martin A.
Niedermeier
a,
Gonzalo
Santoro
b,
Stephan V.
Roth
b and
Peter
Müller-Buschbaum
*a
aTechnische Universität München, Lehrstuhl für Funktionelle Materialien, Physik-Department, James-Franck-Str. 1, 85748 Garching, Germany. E-mail: muellerb@ph.tum.de; Fax: +49 89 289 12473; Tel: +49 89 289 12451
bDeutsches Elektronen-Synchrotron DESY, Notkestr 85, 22603 Hamburg, Germany
First published on 6th November 2013
Abstract
Microfluidic processes which are accompanied with structural changes at the solid–liquid interface are monitored with in situ grazing incidence small angle X-ray scattering (GISAXS) using a micro-focused X-ray beam. Changes of the nanostructure of a film of nanospheres dispersed in a matrix of sodium alginate of algal origin and its cross-linked analogue are followed during flow of water through a microfluidic channel. The initial structural changes caused by the laminar flow in the microfluidic channel are traced and demonstrate the high sensitivity and the possibilities of the in situ GISAXS technique.
In manifold applications in science and technology, polymer and colloidal films are adhered to surfaces of fluid and microfluid systems. These surface layers can comprise on the one hand desired films such as a functional coating. On the other hand unwanted, detrimental films can occur. Flow induced changes of these films can cause problems for the fluid application. Especially on smaller scale, such as in microfluidic devices, problems caused by blocking due to the detachment of aggregates may reduce the lifetime of the fluid devices. In this respect, the investigation of initial structural changes, caused by fluid flow (e.g. of water) is necessary for gaining a complete picture of the morphology and the understanding of the mechanical stability of surface attached systems immersed in flow systems.
In the last years small angle X-ray scattering (SAXS) has been established in microfluidic applications for the investigation of the size, shape, and distribution of nanosized structures in liquid environments.1,2 The achievements of the combination of microfluidic devices and the SAXS techniques are strongly linked to the progress in focusing X-ray optics. With highly brilliant X-ray beams, as offered from 3rd generation synchrotron sources, and micro-focused beam sizes, matching the components of microfluidic devices, small sample volumes can be addressed. Moreover, kinetic investigations with high time resolutions become feasible due to the high brilliance. In these in situ SAXS investigations, samples are probed in specially designed microfluidic devices providing a free liquid jet,3–6 or comprising a microfluidic channel.7–10 These microfluidic techniques have the big advantage that a continuous replacement of the probed volume is assured due to the constant flow of liquid. Thus, any detrimental influences of the X-ray beam on the sample, subsumed as beam damage, are minimized. The high time resolution for kinetic studies in microfluidic experiments is usually achieved by probing different positions along the flow path of the sample with respect to a defined reference point. This reference point in the microfluidic device can be for example the position where the mixing of interacting components occurs. However, with SAXS experiments the bulk volume of the liquid in the microfluidic device is probed, whereas structural information at the solid–liquid interface, e.g. of layers on the substrate or the channel walls, respectively, is difficult to be accessed. For thin layers in transmission geometry the probed volume is simply too small to provide a scattering signal of sufficient statistical relevance. Thus, it is difficult to detect changes of the nanostructure of such layers in microfluidic devices with SAXS. Such structural changes at the solid–liquid interface become accessible with replacing the transmission geometry of the SAXS experiment by a reflection geometry. In the according grazing incidence small angle X-ray scattering (GISAXS) experiment the X-ray beam impinges on the sample under a shallow incident angle well below 1°. Due to a correspondingly enlarged foot print of the X-ray beam on the sample surface, films are probed with high statistical relevance. Moreover, for thin films with GISAXS the full film volume and not only the film surface can be probed. In addition, GISAXS is a non-destructive method with nanometer resolution and consequently particularly well suited to follow structural changes of nano- and mesostructures in situ.11–13 Despite this high potential, applications of GISAXS for structure determination in microfluidic flow experiments are still rare. For example there are experiments, combining GISAXS and microfluidics, focused on the attachment of nanoparticles to a defined substrate mounted in a microfluidic device.14,15 It was shown that gold nanoparticles out of aqueous dispersion preferably attach to one domain of a micro-phase-separated diblock copolymer thin film up to the accumulation of nanowires assembled out of discrete nanoparticles.15 However, the principal questions addressable by the combination of microfluidics and GISAXS are much broader than the investigation of such attachment processes. Many other applications like the kinetics of growth processes of surface-attached particles are conceivable. Moreover the changes of already established nanostructures may be investigated under flow condition.
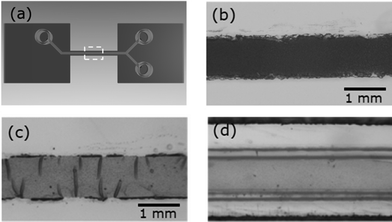
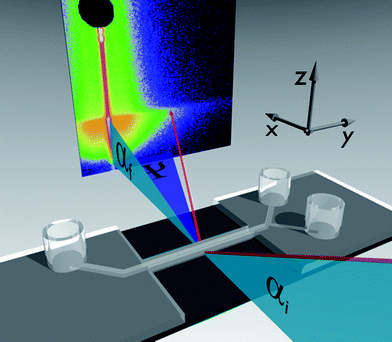
Within the present work we demonstrate the feasibility of such measurements for the first time. The selected model system comprises films consisting of polystyrene nanospheres dispersed in a polyelectrolyte matrix on glass surfaces. In Fig. 1 the preparation of the films and the insertion in the microfluidic cell is subsumed. With adhesive tape a channel groove with a width of about 0.9 mm is established on top of microscope glass slides (Carl Roth, 76 × 26 mm2). Blade coating of a viscous solution of sodium alginate of algal origin (Sigma Aldrich) with dispersed polystyrene spheres of 105 nm diameter (microparticles GmbH) (Fig. 1(a)) is performed. After drying at ambient conditions and removing the adhesive tape, films in narrow stripes of 2 cm length with a width below 1 mm and a film thickness of about 7 μm remain on top of the glass substrate (Fig. 1(b)). A second type of sample is prepared by rinsing the dry films with a 1 M solution of CaCl2 to achieve physical cross-linking with divalent calcium ions and subsequent drying at ambient conditions. The cross-linking process with CaCl2 solution has to be applied to dry sodium alginate films as otherwise no homogeneous films are obtained. Both type of films fit into the channel of our microfluidic cell as it provides a channel with a width and height of 1 mm as sketched in Fig. 1(c). The microfluidic device used in our study is based on a setup developed for GISAXS experiments.14 It comprises two inlet channels which meet in a Y-shaped intersection that can be used for mixing experiments (Fig. 2(a)). The inlets and the outlet are equipped with Luer connectors. The cell consisting of a cyclic olefin based polymer is transparent for visible light and has a low absorption of X-rays for the applied wavelength. The central part of the channel consists of thin walls. A cross section of the channel with inner dimensions 1 mm × 1 mm is given and a thickness of wall of 0.5 mm in Fig. 1(c). As the X-ray beam has to transmit the channel walls in the GISAXS experiment the thickness of the walls has been chosen as small as possible, still providing the necessary mechanical stability. In Fig. 2(b) the top view of an alginate film with dispersed nanospheres on top of a glass slide is shown. The cross-linked films show some wrinkles (Fig. 2(c)), which disappear after the exposure to water in the microfluidic channel as observed with optical microscopy through the microfluidic device after a flow experiment (Fig. 2(d)). Leak tightness of the microfluidic device is assured by mechanically clamping the microfluidic cell to the substrate. It is crucial that no adhesive sealant is used as it would strongly influence the scattering signal, e.g. by a strong increase of the background signal. The GISAXS experiments were performed at the MiNaXS instrument P03 (ref. 16) of the synchrotron PETRA III at DESY, Hamburg, Germany. The wavelength λ = 0.109 nm and a sample-to-detector distance DSD = 3705 mm with an incident angle αi = 0.45° were used. A moderately micro-focused X-ray beam with a size of 31 × 22 μm2 (h × v) leads to a footprint of the beam closely matching the width of the microfluidic channel. The GISAXS signal was recorded with a Pilatus 300k area detector from DECTRIS Ltd. (Baden, Switzerland). In Fig. 3 the GISAXS measurement geometry together with the microfluidic device is shown. The X-ray beam hits the surface under the incident angle αi. Due to the shallow angle the foot print on the sample is enlarged and the complete width of the film inside the channel is illuminated. The scattered intensity is recorded on the two-dimensional detector with the exit angle being αf and the out-of plane angle Ψ. The micro-focused beam allows for scanning along the y-direction of the channel by moving the microfluidic device while fluid flows through the channel. The scanning experiment needs careful alignment to assure a constant incident angle and footprint that stays centered with respect to the width of the channel for the whole scan range of up to 20 mm along the channel in y-direction (Fig. 3). The response of an initially dry film to laminar flow was probed in such a way that the flow direction was opposite to the scan direction of the microfluidic device. A flow of water was installed by a syringe pump (nemesys, Cetoni GmbH) with 0.01 ml min−1 which corresponds to 10 mm min−1 within the microfluidic channel with our channel geometry. The scanning of the microfluidic cell is not continuous but stepwise adopted to the X-ray beam size. In this experiment, X-ray exposures for 1 s in step widths of 125 μm were recorded. This means that the temporal measurements are combined with a change in position. This procedure is chosen to address structural changes caused solely by the flow of water and not by the intense incident X-ray beam as it would be expected for longer illumination times on one particular spot on the sample. From the subsequent individual 2D GISAXS data vertical cuts at Ψ = 0 are composed (Fig. 4). In Fig. 4(a) and (b) distances of 5 mm along the channel in y-direction are covered. The experiment starts with a dry film and the liquid begins to fill the channel before laminar flow is established. The first frame at which the X-ray beam hits the channel with its cross-section completely filled with liquid is observed by the distinct drop in intensity due to absorption of the X-ray beam by the liquid. Close to the full front of liquid, which fills the channel, the individual frames (before the white arrow, around t = 30 s) correspond not necessarily to a completely dry film. As the film is more hydrophilic than the channel walls of the microfluidic device a precursor of liquid wets the surface of the film and initiates structural changes of the film even before the full cross-section of the channel is filled. From the individual 2D GISAXS data horizontal line cuts are performed as well. These cuts are taken at the critical angle of total external reflection of polystyrene for the given X-ray wavelength, which is a material sensitive value.17 The resulting curves for both of the alginate films with dispersed nanospheres are shown in Fig. 5. The dry films show two distinct shoulders (black curves in Fig. 5(a) and (b), marked with blue arrows). In Fig. 5 the curves show from bottom to top the evolution of the scattered intensity during the in situ microfluidic experiment. The first light grey curves represent the situation where the channel is completely filled for the first time, as noticed by the distinct drop in intensity. It becomes clear that a structural change of the film does not only develop when the channel is completely filled (light grey curves in Fig. 5) but already starts before (dark grey curves in Fig. 5). For the sodium alginate film (Fig. 5(a)) the precursor of liquid, which wets the film, smoothens the two distinct structural features. Immediately upon water contact one shoulder transforms into a more prominent peak, indicating a loss of small scale structures. Finally, as sodium alginate is water soluble, a complete dissolution of the film occurs on longer timescales. For a film which is physically cross-linked with calcium ions the process is slowed down. The precursor of liquid does not lead to an immediate smoothening or structural change. Only at full water contact one shoulder transforms into a reasonably prominent peak, witnessing the loss of small scale structures. With ongoing flow of water the scattering curves further transform as seen by a shift of the peak positions towards smaller qy values. These changes are due to a coarsening of structures (indicated by the light blue line in Fig. 5(b)). This corresponds to the swelling of the physically cross-linked film increasing the distance of polystyrene spheres in the alginate matrix. The shown experiments (Fig. 5) cover one minute of microfluidic flow upon first water contact, at which the structure already changes although a mechanically stable film remains as shown in Fig. 2(d).
The identification of extremely small changes of the structure of thin films during an in situ microfluidic experiment on one hand offers the possibility to tune the structure of nanoparticle films by varying the type of liquid, flow rates and the duration of laminar flow. On the other hand the experimental set-up gives the opportunity to investigate the structural changes that precede the detachment of films and nanoparticle aggregates. In combination with an observation on longer time and larger size scale (e.g. with optical microscopy) a complete description of detachment processes will be accessible.
Conclusions
With the presented example of the combination of microfluidics and GISAXS the possibilities of such experimental approach are highlighted by detecting the initial changes of the structure of a film consisting of nanospheres embedded in a polymer matrix upon continuous flow of water. The experimental technique allows for the investigation of attachment processes and layer formations as well as for the investigation of the detachment of unwanted detrimental films. Thus, a technique with sensitivity to changes of nanostructures is available for research with microfluidic devices, which can probe modifications due to aging or degradation of layers and coatings in the microfluidic cell.Acknowledgements
This work has been financially supported by the BMBF (grant number 05K10WOA). M. P. gratefully acknowledges the financial support by the National Research Fund, Luxembourg cofunded under the Marie Curie Actions of the European Commission (FP7-Cofund AFR-PDR 2010-2, 1036107). GISAXS measurements were carried out at the synchrotron light source PETRA III at DESY. DESY is a member of the Helmholtz Association (HGF).Notes and references
- L. Pollack, M. W. Tate, N. C. Darnton, J. B. Knight, S. M. Gruner, W. A. Eaton and R. H. Austin, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 10115 CrossRef CAS.
- L. Pollack, M. W. Tate, A. C. Finnefrock, C. Kalidas, S. Trotter, N. C. Darnton, L. Lucio, R. H. Austin, C. A. Batt, S. M. Gruner and S. G. J. Mochrie, Phys. Rev. Lett., 2001, 86, 4962 CrossRef CAS.
- B. Marmiroli, G. Grenci, F. Cacho-Nerin, B. Sartori, E. Ferrari, P. Laggner, L. Businaro and H. Amenitsch, Lab Chip, 2009, 9, 2063 RSC.
- J. Polte, R. Erler, A. F. Thünemann, S. Sokolov, T. T. Ahner, K. Rademann, F. Emmerling and R. Kraehnert, ACS Nano, 2010, 4, 1076 CrossRef CAS PubMed.
- M. E. Brennich, J. F. Nolting, C. Dammann, B. Nöding, S. Bauch, H. Herrmann, T. Pfohl and S. Köster, Lab Chip, 2011, 11, 708 RSC.
- M. E. Brennich and S. Köster, Microfluid. Nanofluid., 2013 DOI:10.1007/s10404-013-1212-y.
- S. M. Taheri, S. Fischer, M. Trebbin, S. With, J. H. Schröder, J. Perlich, S. V. Roth and S. Förster, Soft Matter, 2012, 8, 12124 RSC.
- R. Stehle, G. Goerigk, D. Wallacher, M. Ballauf and S. Seiffert, Lab Chip, 2013, 13, 1529 RSC.
- B. Weinhausen and S. Köster, Lab Chip, 2013, 13, 212 RSC.
- M. Trebbin, D. Steinhauser, J. Perlich, A. Buffet, S. V. Roth, W. Zimmermann, J. Thiele and S. Förster, Proc. Natl. Acad. Sci. U. S. A., 2013, 112, 550 Search PubMed.
- K. Vegso, P. Siffalovic, M. Weis, M. Jergel, M. Benkovicova, E. Maikova, L. Chitu, Y. Halahovets, S. Luby, I. Capek and A. Satka, Phys. Status Solidi A, 2011, 208, 2629 CrossRef CAS.
- J. Perlich, M. Schwartzkopf, V. Körstgens, D. Erb, J. F. H. Risch, P. Müller-Buschbaum, R. Röhlsberger, S. V. Roth and R. Gehrke, Phys. Status Solidi RRL, 2012, 6, 253 CrossRef CAS.
- M. Schwartzkopf, A. Buffet, V. Körstgens, E. Metwalli, K. Schlage, G. Benecke, J. Perlich, M. Rawolle, A. Rothkirch, B. Heidmann, G. Herzog, P. Müller-Buschbaum, R. Röhlsberger, R. Gehrke, N. Stribeck and S. V. Roth, Nanoscale, 2013, 5, 5053 RSC.
- J.-F. Moulin, S. V. Roth and P. Müller-Buschbaum, Rev. Sci. Instrum., 2008, 79, 015109 CrossRef PubMed.
- E. Metwalli, J.-F. Moulin, J. Perlich, W. Wang, A. Diethert, S. V. Roth and P. Müller-Buschbaum, Langmuir, 2009, 25, 11815 CrossRef CAS PubMed.
- A. Buffet, A. Rothkirch, R. Döhrmann, V. Körstgens, M. M. Abul Kashem, J. Perlich, G. Herzog, M. Schwartzkopf, R. Gehrke, P. Müller-Buschbaum and S. V. Roth, J. Synchrotron Radiat., 2012, 19, 647 CrossRef CAS PubMed.
- P. Müller-Buschbaum, Anal. Bioanal. Chem., 2003, 376, 3 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |