Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionic liquids are compatible with on-water catalysis†
Kaitlin D.
Beare
,
Alexander K. L.
Yuen
,
Anthony F.
Masters
,
Thomas
Maschmeyer
and
Christopher S. P.
McErlean
*
School of Chemistry F11, University of Sydney, Sydney 2006, Australia. E-mail: christopher.mcerlean@sydney.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 3970
First published on 5th August 2013
Abstract
A major limitation of on-water catalysis has been the need for liquid reactants to enable emulsification. We demonstrate that ionic liquids are compatible with on-water catalysis, enabling on-water catalysed reactions for otherwise unreactive solid–solid systems. The unique solvation properties of ionic liquids dramatically expands the scope of on-water catalysis.
The social imperative for sustainability has led to a re-evaluation of traditional chemical methods, and provided impetus for a move towards green chemistry. Solvent replacement and catalysis have been identified as complementary approaches that may help mitigate the impact of chemical synthesis.1 Possessing negligible vapour pressures, ionic liquids are solvents that can theoretically be recycled indefinitely, minimizing waste generation.2,3 Increasing the green credentials of ionic liquids are their unique solvation properties that allow for a wide range of otherwise insoluble materials, such as biomass, to participate in chemical transformations.4–6 Aside from ionic liquids, water is perhaps the most obvious green solvent. It has no toxicity, no flammability, and is readily available, albeit in low purity (e.g. seawater). Crucially, water can function as more than just a solvent. In 2005 Sharpless observed that the rate of reaction between certain insoluble organic compounds increased when the reactions were performed as aqueous emulsions.7 Driven by the unique reactivity of interfacial water,8,9 this mode of reactivity is known as on-water catalysis.10,11
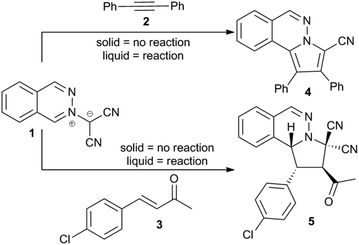
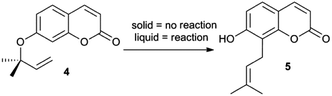
A factor limiting the widespread application of on-water chemistry is the requirement for at least one reagent to be liquid in order to generate the oil-in-water emulsion that is critical for catalysis. For example, Butler and co-workers12 reported that when the dipolar cycloadditions involving phthalazinium-2-dicyanomethanide (1) were performed at temperatures below the melting points of the dipolarophiles 2 and 3 (Scheme 1), there was essentially no reaction. At temperatures above their melting points, 2 and 3 formed aqueous emulsions and the reaction gave the products in high yield. We recently reported similar trends for the unimolecular aromatic Claisen rearrangement (Scheme 2).13 The insoluble compound 4 failed to undergo rearrangement until it was converted into a liquid. We wondered if ionic liquids would serve as the liquid phase and still allow on-water catalysis to occur. Given that the ion pairs could themselves interact with the interface, it was not obvious that ionic liquids would be compatible with interfacial reactivity. We now detail initial outcomes of our investigations.
 | ||
| Scheme 1 Butler's solid vs. liquid dipolar cycloadditions. | ||
 | ||
| Scheme 2 Solid vs. liquid aromatic Claisen rearrangement. | ||
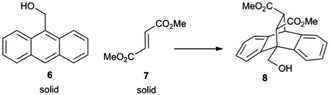
Our first efforts focussed on the Diels–Alder cycloaddition of anthracene-9-carbinol (6). Compound 6 is a solid with only sparing aqueous solubility, but one that is known to undergo a hydrogen-bond-accelerated cycloaddition.14,15 As detailed in Table 1, compound 6 does not participate in a thermal Diels–Alder reaction with solid dimethyl fumarate (7) under neat conditions at 80 °C (entry 1). This removes any contribution of the background thermal reaction to the observed yields of subsequent cycloadditions. When 6 and 7 were added to water and gently stirred (conditions we have previously termed ‘at-water’8) the solid remained in contact with the walls of the reaction vessel and no reaction occurred (entry 2). When the mixture was vigorously stirred in the manner commonly employed to generate an emulsion for on-water reactions (entry 3), no emulsion was formed and the solid remained visible against the surface of the vessel. Unsurprisingly, no reaction occurred. However, when the reagents were dissolved in either non-polar or polar, protic organic solvents (entries 4 and 5) the reaction smoothly gave the desired cycloadduct. This demonstrates that it is not an inherent lack of reactivity that stops the two compounds from reacting under neat or on-water conditions, but rather it is a consequence of their physical state. When the substrates were dissolved in the common ionic liquid [BMIM][NTf2]16 the reaction proceeded sluggishly (entry 6). Could we add a small amount of solvent to the mixture of solid reagents to dissolve them and provide for emulsion formation? The answer is yes. Adding a small amount of an organic solvent to the reaction mixture (entry 7) enabled emulsion formation and the cycloaddition occurred. Of course, it may be that the product was formed only in the organic solvent and that interfacial water played no role. In any event, the addition of organic solvents to generate the emulsion is unsatisfactory as it does not correspond to the ethos of solvent replacement. In contrast, the addition of ionic liquid to the solid reagents (entry 8) not only allowed for easy emulsion formation, but resulted in a much higher level of conversion than was seen in the ionic liquid itself. The cause of this acceleration is not merely physical in origin. Generation of an emulsion using [BMIM][NTf2] and hexadecane (entry 9) gave conversion rates that corresponded to the background reaction occurring in the ionic liquid phase. The cycloaddition of the solid reagents 6 and 7 is accelerated when the reaction is performed as an ionic liquid-in-water emulsion. Ionic liquids are compatible with on-water catalysis.
|
|
||
|---|---|---|
| Entry | Solventa | Conversionb (%) |
| a Reaction conditions: 6 (0.5 mmol), 7 (0.5 mmol) solvent (4 mL), co-solvent (0.5 mL), 80 °C, 16 h. b Based on 1H NMR integration. | ||
| 1 | Neat | 0 |
| 2 | At-water | 0 |
| 3 | On-water | 0 |
| 4 | Toluene | 66 |
| 5 | n-Butanol | 76 |
| 6 | [BMIM][NTf2] | 27 |
| 7 | On-water–toluene | 47 |
| 8 | On-water–[BMIM][NTf2] | 61 |
| 9 | Hexadecane–[BMIM][NTf2] | 31 |
The amount of ionic liquid used to generate the emulsion displays an effect on the efficacy of catalysis. With too little ionic liquid the reagents will not necessarily be emulsified and reaction would occur through Butler's phase transitioning mechanism.12 On the other hand, too much ionic liquid would result in reagent dilution and reduce the likelihood of interaction with interfacial water. After experimentation (see ESI†), we discovered that an approximately 10% v/v ionic liquid-in-water emulsion leads to optimal results, but that the on-water effect is observed at substantially lower concentrations of ionic liquid, which may be beneficial for large scale reactions.
If the ionic liquid is merely acting as a ‘liquefier’ in these on-water reactions then it ought to exert no influence on reactions that already contain a liquid reagent. This is indeed the case. As detailed in Table 2, the Diels–Alder reaction between the liquid diene 9 and the solid dienophile 7 is accelerated on-water (compare entry 1 with entries 2–6). Performing the reaction in an ionic liquid-in-water emulsion gave the same conversion as the reaction performed without the ionic liquid (entries 1 and 7). The ionic liquid had no influence on the rate.
The beneficial impact of ionic liquids lies not in using them as substitutes for organic solvents, but in harnessing their unique properties to dissolve typically insoluble compounds. As such we examined the dipolar cycloaddition between the highly insoluble phthalazinium compound 1 and various dipolarophiles.
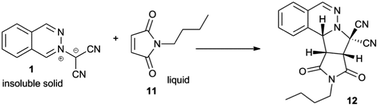
As shown in Table 3, the solid–liquid dipolar cycloaddition between 1 and the liquid phthalimide 11 was monitored at short and long reaction times. At short reaction times the level of conversion correlates with the solubility of compounds 1 and 11. Whilst it is not visible to the eye, compound 1 must have some solubility in the liquid dipolarophile 11 such that the cycloaddition occurs under neat reaction conditions (entry 1). Although 1 is not soluble in water,12 compound 11 is slightly soluble, such that when the reaction is performed at-water (entry 2) some of the dipolarophile dissolves into the aqueous phase leaving less to function as a solvent that facilitates the neat cycloaddition. Consequently, the level of conversion decreases appreciably. The same trend is apparent for organic solvents (entries 3 and 4) in which 11 is soluble, but 1 is not. Both compounds 1 and 11 are soluble in [BMIM][NTf2] (entry 5) and it is here that the rate of the background reaction can be accurately assessed. The scene is now set to ascertain whether or not the reaction is susceptible to on-water catalysis. Attempts to generate an emulsion by vigorous stirring of the reactants in the presence of water (entry 6) failed due to the low solubility of 1.
|
|
|||
|---|---|---|---|
| Entry | Solventa | Conversion at 20 minb,c (%) | Conversion at 6 hb (%) |
| a Reaction conditions: 1 (0.5 mmol), 11 (0.5 mmol) solvent (4 mL), co-solvent (0.5 mL), rt. b Based on 1H NMR integration. c Only the endo isomer was observed. | |||
| 1 | Neat | 33 | 84 |
| 2 | At-water | 24 | 53 |
| 3 | Toluene | 19 | 78 |
| 4 | Ethanol | 21 | 85 |
| 5 | [BMIM][NTf2] | 52 | 79 |
| 6 | On-water | 39 | 88 |
| 7 | On-water–toluene | 23 | 74 |
| 8 | On-water–[BMIM][NTf2] | 79 | 100 |
The rate of conversion mirrored the neat reaction (compare entries 1 and 6). Similarly, employing water–organic mixtures (entry 7) failed to provide an oil-in-water emulsion due to the insolubility of 1. The presence of the organic phase only aided dissolution of 11 and consequently the level of conversion mirrored that of the organic solvent (entry 3). Strikingly, the ionic liquid-in-water emulsion generated with [BMIM][NTf2] led to a large increase in conversion (compare entries 5 and 8). The enhanced solubilising ability of the ionic liquid enabled a previously inaccessible on-water catalysed transformation to be conducted under exceedingly mild conditions.
The reaction between the insoluble 1 and a solid dipolarophile 13 gave similar results (see Table 4). Of note is the observation that low purity water (i.e. sea water) can be used in the process with little effect on catalysis (entry 8). The magnitude of the on-water effect was evident in that the reactions of the imides 11 and 13 conducted as ionic liquid-in-water emulsions reached completion in ca. 80 min, whereas the on-water reactions and the reactions in organic solvents required prolonged reaction times of 12–24 hours (see ESI†).17
|
|
||
|---|---|---|
| Entry | Solventa | Conversion at 20 minb,c (%) |
| a Reaction conditions: 1 (0.5 mmol), 13 (0.5 mmol) solvent (4 mL), co-solvent (0.5 mL), rt. b Based on 1H NMR integration. c Only the endo isomer was observed. | ||
| 1 | Neat | 0 |
| 2 | At-water | 0 |
| 3 | Toluene | 10 |
| 4 | Ethanol | 9 |
| 5 | [BMIM][NTf2] | 32 |
| 6 | On-water | 0 |
| 7 | On-water–toluene | 15 |
| 8 | On-water–[BMIM][NTf2] | 64 |
| 9 | On-sea water–[BMIM][NTf2] | 52 |
Our attention then turned to the most challenging scenario, which was highlighted in Scheme 1. When both reactants are solids with low solubility in water and traditional solvents, then the only recourse has been the thermal liquefaction of at least one of the reagents to access the on-water mode of catalysis. As shown in Scheme 3, the ionic liquid-on-water reaction of 1 and 3 gave the cycloadduct 5 in moderate yield at 40 °C. The reaction was performed slightly above ambient temperature to allow accurate measurement of endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo ratios.17 There was no increase in endo selectivity for this reaction, confirming the on-water nature of the process (see ESI†).18 This result represents a significant advance on existing, energy intensive methods.
exo ratios.17 There was no increase in endo selectivity for this reaction, confirming the on-water nature of the process (see ESI†).18 This result represents a significant advance on existing, energy intensive methods.
 | ||
| Scheme 3 Dipolar cycloaddition of insoluble solids. | ||
Given that emulsion formation is an absolute requirement for on-water catalysis, the emulsifying properties of the ionic liquids ought to exert an effect on the level of conversion.19 As expected, water immiscible ionic liquids facilitate on-water chemistry, whereas water miscible ionic liquids do not (Table 5).
| Entry | Ionic liquida | Water miscibility | Conversion in ILb (%) | Conversion on-IL–waterb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), 13 (0.5 mmol) solvent (4 mL), co-solvent (0.5 mL), rt. b Based on 1H NMR integration. | ||||
| 1 | [EMIM][FAP] | Immiscible | 10 | 18 |
| 2 | [BMIM][NTf2] | Immiscible | 32 | 64 |
| 3 | [EMIM][B(CN)4] | Immiscible | 77 | 98 |
| 4 | [HMIM][BF4] | Miscible | 45 | 6 |
| 5 | [BMIM][BF4] | Miscible | 25 | 9 |
In summary, we have shown for the first time that ionic liquids are compatible with on-water catalysis. Due to the unique solubilizing properties of ionic liquids, this opens new vistas for ionic liquid-on-water catalysis.
This work was funded by the Australian Research Council (DP120102466).
Notes and references
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- M. M. Hossain and L. Aldous, Aust. J. Chem., 2012, 65, 1465–1477 CrossRef CAS.
- M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS.
- J. K. Beattie, C. S. P. McErlean and C. B. W. Phippen, Chem.–Eur. J., 2010, 16, 8972–8974 CrossRef CAS.
- K. D. Beare and C. S. P. McErlean, Org. Biomol. Chem., 2013, 11, 2452–2459 CAS.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS.
- R. N. Butler, A. G. Coyne and E. M. Moloney, Tetrahedron Lett., 2007, 48, 3501–3503 CrossRef CAS.
- K. D. Beare and C. S. P. McErlean, Tetrahedron Lett., 2013, 54, 1056–1058 CrossRef CAS.
- D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816–7817 CrossRef CAS.
- R. Breslow, U. Maitra and D. Rideout, Tetrahedron Lett., 1983, 24, 1901–1904 CrossRef CAS.
- H. Deng, C. Yuan, J. He and J.-P. Cheng, J. Org. Chem., 2012, 77, 7291–7298 CrossRef CAS.
- R. N. Butler, A. G. Coyne, W. J. Cunningham and L. A. Burke, J. Chem. Soc., Perkin Trans. 2, 2002, 1807–1815 RSC.
- R. N. Butler, A. G. Coyne, W. J. Cunningham and E. M. Moloney, J. Org. Chem., 2013, 78, 3276–3291 CrossRef CAS.
- P. J. Scammells, J. L. Scott and R. D. Singer, Aust. J. Chem., 2005, 58, 155–169 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional rate studies, experimental details and compound characterisation. See DOI: 10.1039/c3cc44150d |
| This journal is © The Royal Society of Chemistry 2013 |