One-pot synthesis in polyamines for preparation of water-soluble magnetite nanoparticles with amine surface reactivity†
Haiou
Qu
ab,
Hui
Ma
ab,
Aurélien
Riviere
c,
Weilie
Zhou
b and
Charles J.
O'Connor
*ab
aDepartment of Chemistry, University of New Orleans, 2000 Lakeshore Dr, New Orleans, LA, USA
bAdvanced Materials Research Institute, University of New Orleans, 2000 Lakeshore Dr, New Orleans, LA, USA. E-mail: coconnor@uno.edu.; Fax: +504-280-3185; Tel: +504-280-6848
cChemistry Department, University of Bordeaux, Cours de la Liberation, Talence, France
First published on 13th January 2012
Abstract
Magnetite nanoparticles with hydrophilic surface coating are prepared in polyamine solvents. The resulting products are highly stable in polar solvent. The surface amine groups are available for secondary reactions.
Superparamagnetic iron oxide nanoparticles (SPIONs) have received attention mostly due to their potential to stimulate applications in clinical areas. Numerous studies have been reported on using SPIONs for target delivery, magnetic resonance imaging (MRI), hyperthermia and magnetic separation.1 The majority of these studies adopted thermal decomposition of several iron precursors in non-polar solvent as the method to prepare SPIONs because of the high degree of uniformity, crystallinity and magnetic properties that can be achieved by this route.2 However, the resulting nanoparticles (NPs) are hydrophobic on the surface and capped by monofunctional ligands which are unable to sustain a secondary reaction for further modification. Consequently, these NPs are usually treated with sophisticated room temperature ligand exchange reaction to modify the surface moiety with desired functionalities.3 Although co-precipitation and microemulsion methods can offer NPs with good solubility in aqueous solution,4 a relatively lower degree of control over the size distribution and crystallinity still limit their practical applications.
Several high-temperature synthetic approaches have been developed to prepare water-soluble SPIONs by performing the synthesis in polar solvent at elevated temperature.5 Not only can these high boiling point polar solvents (i.e.diethylene glycol and 2-pyrrolidone) provide a wide operating-temperature range, they also act as reducing agents and coordinating ligands that can effectively stabilize the NPs during the reaction and prevent aggregation.6 Despite interest in direct preparation of uniform water-soluble NPs, the reactivity and availability of the surface functional groups of ligand shell remain an issue, which hinders the subsequent functionalization step for designing complicate multifunctional agent for clinical diagnostic and treatment.
Besides carboxylates,7phosphates,8 and sulfates,2aprimary amines are also known to bind to the surface of NPs due to their coordinating ability with many transition metals.2c,9 While ligand exchange reactions and hydrothermal synthesis of iron oxide NPs using hydrophilic molecules containing amine functionalities as ligand have been reported,2a,10 the colloidal chemistry using polyamines as solvent and stabilizing agents is unexplored, and little attention has been paid to their interesting properties such as high boiling point, water-solubility and reduction abilities. Additionally, amine groups are also well-known for their reactivity in bioconjugation chemistry. A variety of organic molecules are able to couple with amine-containing molecules and give a stable amide or secondary amine bond in a rapid and high yield reaction.11 Herein, we report a one-pot synthesis method, using polyamine as both solvent and stabilizing agents to prepare water-soluble amine functionalized Fe3O4 NPs.
Fig. 1a is a typical transmission electron microscopy (TEM) image of as-prepared Fe3O4 NPs. The narrowly distributed NPs with an average size of 7.4 nm (SD = 9.5%) can be easily achieved by refluxing iron source in polyamine without any size selection process (see ESI†). Synthesis conditions such as concentration of the precursors, heating rate were varied to understand the formation of the NPs. It is suggested that mild heating ramp is important to achieve narrow size distribution while rapidly heating the system from room temperature to reflux (20 °C min−1) would result in NPs with broad size distribution (Fig. S1†). Uniform Fe3O4 NPs with larger diameter can be accomplished via a seed-mediated growth method. By mixing the seeds, 7.4 nm NPs, with more iron precursors, the average diameter is increased to 9.8 nm (SD = 12.2%). Following the same procedure, 12 nm or larger NPs can be prepared. The histogram of each size group of the Fe3O4 NPs is given in Fig. 1 and examination of this figure shows that different sized Fe3O4 NPs are all quasi-spherical with small size deviations.
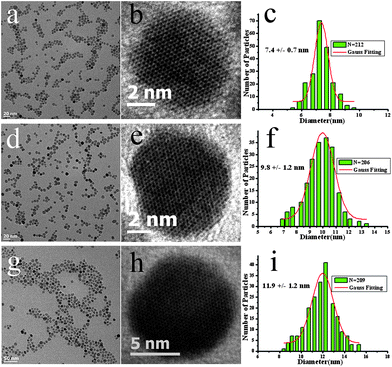 | ||
| Fig. 1 TEM images and size statistics for Fe3O4 NPs: (a,d,g) low resolution, (b,e,h) high resolution, (c,f,i) size distribution of different sized Fe3O4 NPs. | ||
High resolution TEM (Fig. 1) and XRD studies (Fig. S2†) confirm good crystallinity and phase purity of the as prepared NPs, and the XRD pattern matches well with the standard bulk magnetite (JCPDS no 19-0629). Previous studies have shown that, metal precursors can be reduced and yield nucleation in the present of primary amines.9,12 Similar results are observed in the present study, indicating a vital role of hydrophilic TETA in the synthesis of iron oxide NPs. Although, a clear mechanism is not yet fully understood, polyamines are believed to provide a reductive environment to partially reduce the iron precursors and facilitate the nucleation and subsequent growth period.
The magnetic properties were studied in a Quantum Design SQUID magnetometer (MPMS XL-7) and Fe3O4 NPs of all sizes exhibit superparamagnetism at 300 K (Fig. S3†). The weight percentages of Fe3O4 NPs in different sizes were acquired by TGA and confirmed by ICP-AES. After normalizing to the magnetic core, the saturation magnetizations of pure Fe3O4 are 75.8 emu g−1, 79.5 emu g−1 and 82.0 emu g−1 respectively, which are very close to the value of magnetite NPs in similar size reported in the literature.2c,12,13
The surface composition of as-synthesized NPs was studied by FT-IR and XPS. As shown in Fig. S4, as-prepared NPs give similar spectrum as neat TETA. The peak at 1600 cm−1 is assigned to N–H bending, and the peaks in 2800 cm−1 to 3000 cm−1 region are corresponded to the symmetric and asymmetric CH2 stretches, which indicate that as-prepared Fe3O4 NPs are coated with polyamine molecules (see ESI† for detailed calculation). As reported in the literature, the binding energy of N 1s will shift to lower value if a chemical bond is formed between the nitrogen and metal elements.5b From XPS study (Fig. S5†), we observed that the binding energy of N 1s was sifted to 369.9 eV, indicating a direct coordination of nitrogen with iron, which directly prove the presence of TETA as capping ligand on the surface of NPs. The TETA protected NPs can be easily dispersed in polar solvents such as alcohols, DMSO and water or buffer solutions for several months without any precipitates. The small polydispersity from DLS measurement (Table S1†) also confirm the good colloidal stability of polyamine coated NPs. Additionally, ascribed to the protonation of multiple amine groups from polyamine molecules, TETA coated NPs are highly positively charged in aqueous solution.
The surface moiety of NPs should not only tolerate different reaction conditions to protect the inner core materials, but also present enough reactivity and availability for secondary modification. As indicated from IR and zeta potential measurement, there are free amine groups dangling near the surface of the TETA coated NPs, which provide possible reaction sites for further modification. We have adopted two pathways to study the surface reactivity. Previous studies have shown that amine-functionalized NPs can be used for noble metal NPs or quantum dots (QDs) attachment.14 Herein, negatively charged gold crystals with an average diameter of 3 nm were used. From TEM study (Fig. 2a), small gold nanocrystals are uniformly attached onto the surface of Fe3O4 NPs. No observable change was found in TEM study after a one hour sonication treatment, implying a strong affinity of gold to amine groups. Similarly, QDs can also be decorated to the surface of NPs via their affinity with amines (see ESI†).
 | ||
| Fig. 2 (a) TEM image of gold nanocrystals attached Fe3O4 NPs (inset: EDS scan of Au@ Fe3O4). (b) TEM image of Fe3O4 NPs functionalized with PAA (inset: FT-IR spectrum of Fe3O4 NPs after functionalization with PAA). | ||
Besides their affinity with transition metal elements, amine groups are also expected to be reactive in organic reactions. For example, under the activation of zero length linker EDC, biocompatible polymer polyacrylic acid (PAA) was coupled to the surface of magnetite NPs viaamine side. The significant changes in the zeta potential values and weight percentage in organic layer from ICP measurement suggest a successful coupling. Additionally, from FT-IR study (Fig. 2b), the two intense peaks at 1581 cm−1 and 1414 cm−1 are assigned to asymmetric and symmetric COO- stretching. The absorption peak corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxylic group is located at 1704 cm−1, which also demonstrates that the conjugation between free amine group and PAA is achieved. TEM studies showed that this functionalization does not cause any effect on the shape or size of the NPs. Biocompatible polymer coating can prolong the circulation time and effectively reduce nonspecific interaction with protein and other biomolecules, which makes the surface modified iron oxide NPs promising candidate as contrast agent in MRI.
O of carboxylic group is located at 1704 cm−1, which also demonstrates that the conjugation between free amine group and PAA is achieved. TEM studies showed that this functionalization does not cause any effect on the shape or size of the NPs. Biocompatible polymer coating can prolong the circulation time and effectively reduce nonspecific interaction with protein and other biomolecules, which makes the surface modified iron oxide NPs promising candidate as contrast agent in MRI.
Many studies have shown that magnetic NPs can be applied in separation of bio-related molecules. Here, the strong binding between biotin and streptavidin was chosen as a model to test the potential of polyamine coated magnetite NPs in bioseparation. Biotin was first conjugated to the as-prepared NPs under similar reaction condition as in PAA conjugation. FT-IR scan shows that a strong amide bond located at 1681 cm−1 (Fig. S6†) appeared after the conjugation. From TEM study, no obvious difference was observed after the coupling of biotin. To test their ability in bioseparation, the biotin conjugated NPs dispersion was added to a PBS buffer containing FITC-labeled streptavidin. After shaking for 30 min, the solid product was collected with a small permanent magnet and the solution was measured. As illustrated in the emission spectra (Fig. S7†), the fluorescent intensity dropped to only 4% of the initial intensity. As a control group, as-prepared Fe3O4 NPs without biotin conjugation were also incubated with FITC-labeled streptavidin for 30 min and collected with a magnet. The fluorescent intensity of the remaining solution still exhibited 80% intensity of the origin (Fig. S8†), indicating that the sharp intensity drop must result from the effective binding of biotin-Fe3O4 NPs with streptavidin rather than non-specific interaction with the proteins. The biotinylated Fe3O4 NPs were also mixed with Cy5 labeled normal mouse IgG, and 90% of the intensity remained after the separation (Fig. S9†), implying a very limited non-specific interaction with irrelevant proteins. The fluorescent images of isolated NP dispersion under UV light (Fig. 3a) gave a direct-viewing of the efficient separation process. We also tested their separation ability in a mixture of different types of proteins. Under identical experimental condition, a mixture of Cy5 labeled normal mouse IgG and FITC-labeled streptavidin was incubated with biotin-Fe3O4 NPs. The separation process (Fig. 3b) caused only 13% decrease of fluorescent intensity in Cy5 while 91% of the FITC-labeled streptavidin was collected during the process. Meanwhile, the fluorescent color of the solution was changed from yellow to red during the separation, which implies that streptavidin are specifically separated by biotin-Fe3O4 NPs.
 | ||
| Fig. 3 (a) Fluorescent images from the solutions 1: before and 2: after mixing with the nanoparticles. Left and middle: biotinylated and as-prepared Fe3O4 NPs with FITC-streptavidin. Right: biotinylated Fe3O4 NPs with Cy5-IgG, (b) fluorescence spectra from the mixture solutions of FITC-streptavidin and normal mouse IgG labeled by Cy5 before and after treating with biotinylated Fe3O4 NPs (inset: fluorescent images showing the separation process. Left: before, middle: after treating with biotinylated Fe3O4, right: redispersed Fe3O4 NPs separated FITC-streptavidin). | ||
We describe a wet chemistry synthesis procedure to prepare narrowly distributed, spherical shaped magnetite NPs with controlled size using polyamines as solvent, reducing and stabilizing agents. The surface moiety not only stabilizes NPs in various polar solvent, they also provide functional groups that are available for either attachment of a different nanomaterial or biomolecules conjugations. Separation of streptavidin from a mixture of protein using biotin linked NP clearly demonstrated the potential of the magnetite NPs prepared by our method in bioseparation applications. A systematic study is under way to understand the synthesis mechanism. We expect these findings will help us extend this method to the synthesis of other transitional metal ferrite nanoparticles.
This work was supported by a research grant from Louisiana Board of Regents contract no. LEQSF (2007–12)-ENH-PKSFI-PRS-04. We thank Dr Baobao Cao for his help in the TEM study and Kai Wang for helpful discussions.
Notes and references
- (a) F. Dilnawaz, A. Singh, C. Mohanty and S. K. Sahoo, Biomaterials, 2010, 31, 3694 CrossRef CAS; (b) N. Nitin, L. E. W. LaConte, O. Zurkiya, X. Hu and G. Bao, JBIC, J. Biol. Inorg. Chem., 2004, 9, 706 CrossRef CAS; (c) K. Hayashi, M. Moriya, W. Sakamoto and T. Yogo, Chem. Mater., 2009, 21, 1318 CrossRef CAS; (d) C. Xu, K. Xu, H. Gu, R. Zheng, H. Liu, X. Zhang, Z. Guo and B. Xu, J. Am. Chem. Soc., 2004, 126, 9938 CrossRef CAS.
- (a) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS; (b) J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS; (c) S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204 CrossRef CAS; (d) K. Woo, J. Hong, S. Choi, H.-W. Lee, J.-P. Ahn, C. S. Kim and S. W. Lee, Chem. Mater., 2004, 16, 2814 CrossRef CAS; (e) P. Guardia, N. Pérrez, A. Labarta and X. Batlle, Langmuir, 2009, 26, 5843 CrossRef.
- (a) H. Gu, Z. Yang, J. Gao, C. K. Chang and B. Xu, J. Am. Chem. Soc., 2004, 127, 34 CrossRef; (b) M. S. Nikolic, M. Krack, V. Aleksandrovic, A. Kornowski, S. Förster and H. Weller, Angew. Chem., Int. Ed., 2006, 45, 6577 CrossRef CAS; (c) M. Lattuada and T. A. Hatton, Langmuir, 2006, 23, 2158 CrossRef; (d) J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228 CrossRef CAS.
- (a) J.-R. Jeong, S.-C. Shin, S.-J. Lee and J.-D. Kim, J. Magn. Magn. Mater., 2005, 286, 5 CrossRef CAS; (b) L. A. Harris, J. D. Goff, A. Y. Carmichael, J. S. Riffle, J. J. Harburn, T. G. St. Pierre and M. Saunders, Chem. Mater., 2003, 15, 1367 CrossRef CAS.
- (a) Z. Li, Q. Sun and M. Gao, Angew. Chem., Int. Ed., 2005, 44, 123 CrossRef CAS; (b) Z. Li, H. Chen, H. Bao and M. Gao, Chem. Mater., 2004, 16, 1391 CrossRef CAS; (c) H. Qu, D. Caruntu, H. Liu and C. J. O'Connor, Langmuir, 2011, 27, 2271 CrossRef CAS.
- (a) W. Cai and J. Wan, J. Colloid Interface Sci., 2007, 305, 366 CrossRef CAS; (b) D. Caruntu, G. Caruntu, Y. Chen, C. J. O'Connor, G. Goloverda and V. L. Kolesnichenko, Chem. Mater., 2004, 16, 5527 CrossRef CAS.
- (a) N. J. Turro, P. H. Lakshminarasimhan, S. Jockusch, S. P. O'Brien, S. G. Grancharov and F. X. Redl, Nano Lett., 2002, 2, 325 CrossRef CAS; (b) T. Bala, B. L. V. Prasad, M. Sastry, M. U. Kahaly and U. V. Waghmare, J. Phys. Chem. A, 2007, 111, 6183 CrossRef CAS.
- (a) C. Yee, G. Kataby, A. Ulman, T. Prozorov, H. White, A. King, M. Rafailovich, J. Sokolov and A. Gedanken, Langmuir, 1999, 15, 7111 CrossRef CAS; (b) M. I. Tejedor-Tejedor and M. A. Anderson, Langmuir, 1990, 6, 602 CrossRef CAS.
- M. J. Polking, H. Zheng, R. Ramesh and A. P. Alivisatos, J. Am. Chem. Soc., 2010, 133, 2044 CrossRef.
- W. Leyu, B. Jie, W. Lun, Z. Fang and L. Yadong, Chem.–Eur. J., 2006, 12, 6341 CrossRef.
- G. T. Hermanson, Bioconjugate Techniques., New York: Academic Press, 1996 Search PubMed.
- Z. Xu, C. Shen, Y. Hou, H. Gao and S. Sun, Chem. Mater., 2009, 21, 1778 CrossRef CAS.
- D. Caruntu, G. Caruntu and C. J. O'Connor, J. Phys. D: Appl. Phys., 2007, 40, 5801 CrossRef CAS.
- (a) X. Teng, D. Black, N. J. Watkins, Y. Gao and H. Yang, Nano Lett., 2003, 3, 261 CrossRef CAS; (b) D. Wang, J. He, N. Rosenzweig and Z. Rosenzweig, Nano Lett., 2004, 4, 409 CrossRef CAS; (c) S. I. Stoeva, F. Huo, J.-S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 15362 CrossRef CAS; (d) J. Kim, J. E. Lee, J. Lee, Y. Jang, S.-W. Kim, K. An, J. H. Yu and T. Hyeon, Angew. Chem., Int. Ed., 2006, 45, 4789 CrossRef CAS; (e) L. Zhang, W.-F. Dong, Z.-Y. Tang, J.-F. Song, H. Xia and H.-B. Sun, Opt. Lett., 2010, 35, 3297 CrossRef CAS; (f) Y. Gao and Z. Tang, Small, 2011, 7, 2133 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedure, XRD, XPS, FT-IR, and results from photoluminescence measurement and magnetic properties measurement. See DOI: 10.1039/c2jm15932e |
| This journal is © The Royal Society of Chemistry 2012 |
