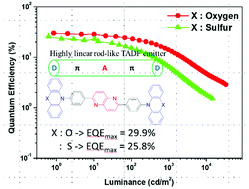
Thermally activated delayed fluorescence (TADF) emitters containing 1,5-naphthyridine as an electron acceptor and phenoxazine and phenothiazine as electron donors, namely, 2,6-bis(4-(10H-phenoxazin-10-yl)phenyl)-1,5-naphthyridine (NyDPO) and 2,6-bis(4-(10H-phenothiazin-10-yl)phenyl)-1,5-naphthyridine (NyDPt), were developed. Because of the linear molecular structures, NyDPO and NyDPt showed high horizontal emitting dipole ratios of 81% and 84%, respectively. Furthermore, NyDPO and NyDPt exhibited TADF characteristics with photoluminescence quantum yields (PLQYs) of 79% and 45%, respectively. In particular, NyDPt showed dual photoluminescence (PL) emission from quasi-axial and quasi-equatorial conformers. However, only quasi-equatorial emission was observed in the organic light-emitting diode (OLED) at low current density, resulting in a high device efficiency despite a low PLQY. OLED devices based on NyDPO and NyDPt exhibited high external quantum efficiencies of 29.9% and 25.8%, and maximum luminance values of 33 540 cd m−2 and 14 480 cd m−2, respectively.