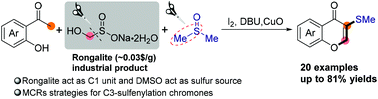
An efficient I2–DMSO reagent system-mediated multicomponent reaction strategy for the synthesis of C3-sulfenylated chromones from o-hydroxyaryl methyl ketones, rongalite, and dimethyl sulfoxide has been developed. Notably, the double C–S bond cleavages of rongalite and dimethyl sulfoxide served as key steps in this smooth transformation, affording the C1 unit and sulfur source for the assembly of C3-sulfenylated chromones. Preliminary mechanistic studies indicated that in situ generated HCHO and (2-(2-hydroxyphenyl)-2-oxoethyl)dimethylsulfonium iodine were probably the key intermediates in this transformation.