Rational utilization of intramolecular and intermolecular hydrogen bonds to achieve desirable electron transporting materials with high mobility and high triplet energy†
Abstract
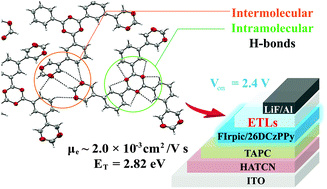
Three new pyrimidine-containing star-shaped compounds, namely, 1,3,5-tri(3-(pyrimidin-5-yl)phenyl)benzene (TPM-TPB), 2,4,6-tris(3-(pyrimidin-5-yl)phenyl)-1,3,5-triazine (TPM-TAZ) and 3,5,6-tris(3-(pyrimidin-5-yl)phenyl)-1,2,4-triazine (TPM-i-TAZ), were synthesized and characterized. These new compounds exhibited favorable electronic affinity (Ea >2.81 eV) and the triplet energy levels (ET) up to ∼2.83 eV. X-ray diffraction analysis of the compounds revealed that the intramolecular and intermolecular C–H⋯N hydrogen bonds of TPM-TAZ resulted in high electron mobility up to 2.0 × 10−3 cm2 V−1 s−1. Using these compounds as the electron transporting materials, the blue phosphorescent organic light-emitting devices showed good performance, with a very low turn-on voltage of 2.4 V, a maximum current efficiency of 26.4 cd A−1, and a maximum power efficiency of 26.9 lm W−1.

 Please wait while we load your content...
Please wait while we load your content...