Reduction and oxidation of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) induced by methylamine (CH3NH2)-containing atmosphere for perovskite solar cells†
Abstract
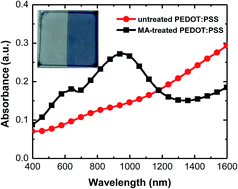
Perovskite solar cells have been attracting a lot of attention because of their high power conversion efficiency and low-cost processing. However, device reproducibility has been a problem. The fabrication atmosphere is regarded as one of the possible reasons. So far, there has been a lack of direct evidence to prove which kind of atmosphere and how the atmosphere affects the device performance. Here, we report that the methylamine (MA, boiling point: −6 °C) that is used to synthesize the methylammonium iodide (MAI) could chemically reduce the PEDOT:PSS hole-transporting layer. After the reduction, a strong absorbance band appears at 400–1100 nm and the conductivity and work function simultaneously decrease. Furthermore, the MA-reduced PEDOT:PSS films are also found to be easily oxidized in air. The reduced work function of the PEDOT:PSS layer leads to poor hole collection and yields low open-circuit voltage, short-circuit current and power conversion efficiency of the perovskite solar cells. Therefore, though the MA vapor-containing fabrication atmosphere is beneficial to the performance of TiO2-based perovskite solar cells in which the bottom electrode collects the electrons, it is detrimental to that of PEDOT:PSS-based solar cells.

 Please wait while we load your content...
Please wait while we load your content...