Modulating the cobalt redox potential through imidazole hydrogen bonding interactions in a supramolecular biomimetic protein-cofactor model†
Abstract
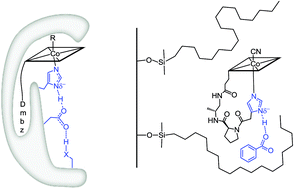
A realistic model for the active site of histidine-on cobalamin@protein complexes is reported and studied under homogeneous and immobilized conditions. Analysis of lower ligand modulation and its influence on the properties of the biomimetic compound are presented. The cofactor attachment by a protein's histidine residue was imitated by covalently linking an artificial imidazole-containing linker to cobyric acid. The resulting intramolecular coordination complex is an excellent structural model of its natural archetype, according to 2D 1H-NMR studies and molecular modeling. The effect of deprotonation of the axially coordinating imidazole ligand – as proposed for natural cofactor complexes – tunes significantly the position of the cathodic peak (ΔV = −203 mV) and stabilizes thereby the CoIII form. Partial deprotonation of the imidazole moiety through hydrogen bonding interactions was then achieved by immobilizing the biomimetic model on hydrophobic C18 silica, which yielded an unprecedented insight on how this class of Cbl-dependent proteins may fine-tune their properties in biological systems.


 Please wait while we load your content...
Please wait while we load your content...