Asymmetric cationic lipid based non-viral vectors for an efficient nucleic acid delivery†
Abstract
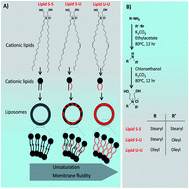
Cationic lipids have been extensively studied for their ability to complex with nucleic acids to condense and consequently deliver them into the cells. However, developing safe and efficient cationic lipids for delivering nucleic acids is still an unmet challenge. Prior structure-activity investigations led to the path to understanding the lipid structure and its transfection efficiency. The trend in the transfection profiles of linker-based lipids is different from linker-less lipids. Influence of unsaturation in the hydrophobic chains has been investigated in linker-based lipids. However, in linker-less lipids, it remains unexplored. Herein, we demonstrate that the designed cationic lipid Lipid S-U with an asymmetric hydrophobic core having one stearyl (18 : 0) and one oleyl chain (18 : 1) showed superior transfection efficiency compared to its symmetric counterparts, Lipid S-S (hydrophobic core comprising of two stearyl chains (18 : 0)), and Lipid U-U (two oleyl chains (18 : 1)), in vitro. Mechanistic studies involving membrane fusogenicity with FACS revealed that liposomes of Lipid S-U have higher fusogenicity (89%) with B16F10 cell membrane than saturated Lipid S-S (66%) and unsaturated Lipid U-U (70%). Endosomal escape studies with confocal microscopy in HEK 293 cells revealed that lipoplexes of Lipid S-U had a higher endosomal escape and released the genetic payload in cytoplasm more efficiently than saturated Lipid S-S and unsaturated Lipid U-U. These cumulative findings support the notion that higher cellular uptake and endosomal escape resulting from fusogenic liposomes of Lipid S-U play a pivotal role in the higher transfection efficiency of asymmetric Lipid S-U.

 Please wait while we load your content...
Please wait while we load your content...