Interactions of omeprazole-based analogues with cytochrome P450 2C19: a computational study†
Abstract
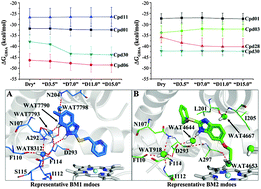
Cytochrome P450 2C19 (CYP2C19) is one of 57 drug metabolizing enzymes in humans and is responsible for the metabolism of ∼7–10% of drugs in clinical use. Recently omeprazole-based analogues were reported to be the potent inhibitors of CYP2C19 and have the potential to be used as the tool compounds for studying the substrate selectivity of CYP2C19. However, the binding modes of these compounds with CYP2C19 remain to be elucidated. In this study, a combination of molecular docking, molecular dynamics (MD), and MM/GBSA calculations was employed to systematically investigate the interactions between these compounds and CYP2C19. The binding modes of these analogues were analyzed in detail. The results indicated that the inclusion of explicit active site water molecules could improve binding energy prediction when the water molecules formed a hydrogen bonding network between the ligand and protein. We also found that the effect of active site water molecules on binding free energy prediction was dependent on the ligand binding modes. Our results unravel the interactions of these omeprazole-based analogues with CYP2C19 and might be helpful for the future design of potent CYP2C19 inhibitors with improved metabolic properties.

 Please wait while we load your content...
Please wait while we load your content...