The first organocopper tetrazole derivative: synthesis and characterization†
Abstract
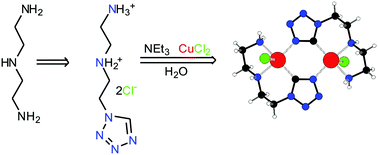
1-(5-Amino-3-azapentyl)tetrazole dihydrochloride (HL·2HCl) was prepared by heterocyclization of diethylenetriamine with triethyl orthoformate and sodium azide followed by treatment with potassium carbonate and hydrochloric acid. The reaction of CuCl2, HL·2HCl and triethylamine (NEt3) in a molar ratio of 1 : 1 : 3 in water was found to generate a novel organometallic tetrazole derivative Cu2L2Cl2. This compound is present as a binuclear centrosymmetric molecular complex, in which C-deprotonated tetrazole L acts as a chelating ligand via the two amino N and tetrazole ring C coordination sites and the two copper atoms are linked together through two tetrazole ring N4–C5 bridges. This complex is the first organocopper tetrazole derivative. When the molar ratio of the reagents in the abovementioned reaction was changed to 1 : 2 : 2, the complex Cu(HL)2Cl2 was formed along with Cu2L2Cl2. Pure Cu(HL)2Cl2 was isolated after reaction of the reagents in a molar ratio of 1 : 3 : 6. The complex Cu(HL)2Cl2 is present as a mononuclear molecular complex, with a chelating coordination mode of HL via the two amino N atoms only. Both complexes as well as HL·2HCl were characterized by single-crystal X-ray analysis.

 Please wait while we load your content...
Please wait while we load your content...