Making C–H bonds with CO2: production of formate by molecular electrocatalysts
Abstract
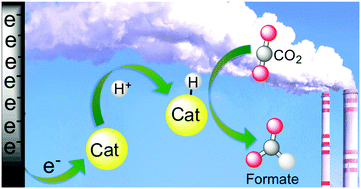
Molecular approaches to the electrocatalytic reduction of CO2 to formate are varied and versatile in their methods. We discuss recent efforts to catalyse this reaction including significant progress made in the last 5 years. This Feature Article begins with a survey of molecular electrocatalysts that produce CO or H2, but have been observed under certain conditions to afford some formate. These examples are included because they provide valuable mechanistic insight for design of catalysts that produce hydrogenated products selectively from CO2. The subsequent discussion describes catalyst properties that favour C–H bond formation with CO2 and this is followed by recent advances that have been made in developing these catalysts. The focus on specific catalyst systems includes recently reported Ir PCP-type pincer complexes and Fe carbonyl clusters, such as [Fe4N(CO)12]−, that selectively produce formate from CO2 in aqueous solution. A discussion of the relevant thermochemical properties of the catalysts in the context of formate production is included.
- This article is part of the themed collection: Celebrating the ChemComm Emerging Investigator Lectureship Winners

 Please wait while we load your content...
Please wait while we load your content...