Structural bases for mechano-responsive properties in molecular gels of (R)-12-hydroxy-N-(ω-hydroxyalkyl)octadecanamides. Rates of formation and responses to destructive strain†
Abstract
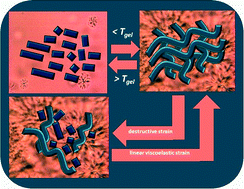
The self-assembly and gelation behavior of a series of (R)-12-hydroxy-N-(ω-hydroxyalkyl)octadecanamides (HS-n-OH, where n = 2, 3, 4 and 5 is the length of the alkyl chain on nitrogen), as well as those of two ‘model’ compounds, N-(3-hydroxypropyl)octadecanamide (S-3-OH) and (R)-12-hydroxy-N-propyloctadecanamide (HS-3), have been investigated in a wide range of liquids. A unique aspect of some of the HS-n-OH gels is the degree and velocity of their recovery of viscoelasticity after the cessation of destructive shear. The recovery times vary from less than one second to hundreds of seconds, depending on the length of the ω-hydroxyalkyl group on nitrogen. The data indicate that the modes and dynamics of aggregation of the gelator molecules from incubation of a sol phase below the gel melting temperature, as analyzed by Avrami and fractal equations, cannot be used to explain the degree and dynamics of the thixotropy: sol-to-gel transformations involve assembly of 0-dimensional objects (i.e., individual gelator molecules) into 1-dimensional fibrils and then into 3-dimensional networks; recovery after mechano-destruction of gels requires only 1-dimensional to 3-dimensional re-assembly or re-association of 3-dimensional spherulitic objects. A model to understand the extreme sensitivity of the thixotropy on the length of the ω-hydroxyalkyl group in the HS-n-OH (which is based upon detailed comparisons among the dynamic properties of the gels, the morphologies of the neat gelators, and the fibrillar networks of the gels) invokes the importance of the cleavage and reformation of H-bonds between fibers at ‘junction zones’ or between spherulitic objects.

 Please wait while we load your content...
Please wait while we load your content...