Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles
Abstract
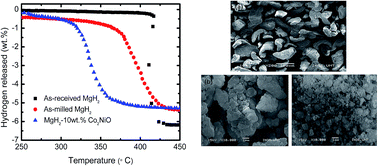
A sample of MgH2 and 10 wt% Co2NiO was prepared by the ball milling technique. The hydrogen storage properties and reaction mechanism of the sample were examined. The temperature-programmed desorption result shows that the addition of 10 wt% Co2NiO to MgH2 exhibited a lower onset desorption temperature of 300 °C, which was decreased to 117 and 70 °C compared to as-received and as-milled MgH2, respectively. The de/rehydrogenation kinetics of MgH2 + 10 wt% Co2NiO showed improvement compared to un-doped MgH2. The results of the Kissinger plot shows that the activation energy for the hydrogen desorption of MgH2 was reduced to about 65.0 and 15.0 kJ mol−1 compared to as-received and as-milled MgH2, respectively. Meanwhile, the X-ray diffraction analysis shows the formation of a new phase of Mg–Co alloy and Co1.29Ni1.71O4 after the dehydrogenation and rehydrogenation process. It is reasonable to conclude that the Co2NiO additive plays a catalytic role through the formation of active species of Mg–Co alloy and Co1.29Ni1.71O4 during the heating process, thus improving the hydrogen storage properties of MgH2.

 Please wait while we load your content...
Please wait while we load your content...