High-molecular weight star conjugates containing docetaxel with high anti-tumor activity and low systemic toxicity in vivo
Abstract
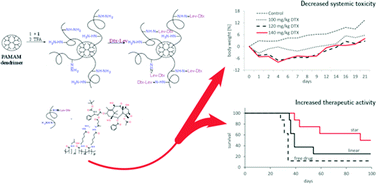
Here we present the polymer conjugates where the core formed by poly(amido amine) dendrimers was grafted with semitelechelic N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers containing docetaxel (DTX) attached by a pH-sensitive hydrazone bond. DTX was derivatized with three different keto acids prior to attachment to the polymer carrier to introduce reactive keto groups into the drug. The therapeutic efficacy of such high-molecular-weight star conjugates is based on: (a) the enhanced permeability and retention (EPR) effect facilitating selective accumulation within solid tumors; (b) pH-controlled release of the drug, thus ensuring faster DTX release in the mildly acidic tumor microenvironment. The star DTX conjugate had a remarkably higher maximum tolerated dose in comparison with free DTX when administered as a single i.v. injection (∼160 mg kg−1vs. 40 mg kg−1 of DTX) in C57BL/6 mice. The star DTX conjugate showed significantly higher antitumor activity than free drug in the EL4 T cell lymphoma growing in syngeneic C57BL/6 mice even when given at the same dose (20 mg kg−1 of DTX eq.). Thus, the star DTX conjugates exert a much higher therapeutic activity and yet a lower systemic toxicity than free DTX.

 Please wait while we load your content...
Please wait while we load your content...