Direct reductive amination of aldehydes with nitroarenes using bio-renewable formic acid as a hydrogen source†
Abstract
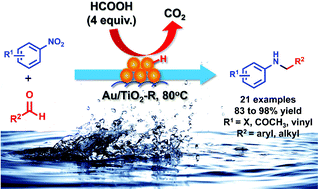
Reductive amination (RA) is one of the most important transformations in organic chemistry. A versatile and sustainable gas-free RA of aldehydes carried out directly with cheaply available nitroarenes using stoichiometric amounts of non-toxic and entirely renewable formic acid (FA) as the terminal reductant is described herein. A single phase rutile titania supported gold (Au/TiO2-R) catalyst is shown to catalyse efficiently this FA-based direct RA in neat water under mild reaction conditions. The broad scope, mild and neutral conditions, together with CO2 and water as environmental harmless byproducts, make this transformation very useful. Moreover, straightforward examples of the direct construction of bioactive heterocyclic compounds containing a benzimidazole motif were achieved through this protocol.

 Please wait while we load your content...
Please wait while we load your content...