Equilibrium and kinetics of aniline adsorption onto crosslinked sawdust-cyclodextrin polymers
Abstract
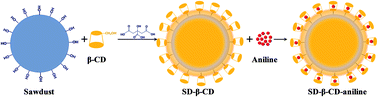
A natural origin adsorbent was prepared by sawdust and beta-cyclodextrin (β-CD) for the removal of an organic compound (aniline) during water pollution accidents. The results of BET, SEM and FTIR analyses indicated that β-CD attaches on the sawdust surface and forms an organic film. Batch tests show that the optimum pH for aniline adsorption is in the range of 4–8. Adsorption equilibrium is achieved in 30 minutes, and the adsorption kinetics follows a pseudo-second-order kinetic model. The Langmuir model appears to fit the isotherm data better than the Freundlich model at 15 °C compared with that at 30 °C and 45 °C. The maximum adsorption capacity is estimated to be 84.03 mg g−1 at 15 °C (R2 > 0.99). The negative value of the standard entropy (ΔHo) and standard free energy (ΔGo) indicates an exothermic and spontaneous nature of the adsorption interaction. Moreover, the results of FTIR suggest that the formation of an inclusion complex between the β-CD and aniline molecules through host–guest interactions enhances the adsorption capability.

 Please wait while we load your content...
Please wait while we load your content...