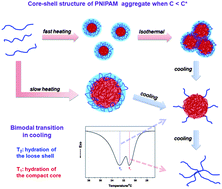
The core–shell structure of a poly(N-isopropylacrylamide) (PNIPAM) collapsed chain conformation is an intermediate conformation formed in the heating process, which was found not only in single-chain solution but also in dilute aqueous solution. However, the relationship between the intermediate structures and thermodynamic properties is still not clear. Herein, the behavior of the bimodal transition, in which two exothermic peaks appear in the DSC cooling curves, has been investigated through different effects of polymer molecular weight, polymer concentration and thermal history. The results show that the higher-temperature peak (T2) is attributed to the hydration of the loose shell while the lower-temperature peak (T1) is ascribed to the further hydration of the compact core. However, the bimodal transition in cooling is observed clearly only when the PNIPAM concentration is less than its overlap concentration (C*). Moreover, it is found that the ratio of the T2 peak can be enhanced either with higher heating rate or less isothermal time at high temperature, since more structures of the loose periphery could be preserved in these cases. For measurements in the cooling–heating cycle, in addition, the result also reveals that the assembled behavior of the core–shell structure is reversible.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...