Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Hydroxy-directed iridium-catalyzed enantioselective formal β-C(sp2)–H allylic alkylation of α,β-unsaturated carbonyls
Sankash
Mitra
 ,
Rahul
Sarkar
,
Rahul
Sarkar
 ,
Aditya
Chakrabarty
,
Aditya
Chakrabarty
 and
Santanu
Mukherjee
and
Santanu
Mukherjee
 *
*
Department of Organic Chemistry, Indian Institute of Science, Bangalore 560 012, India. E-mail: sm@iisc.ac.in; Fax: +91-80-2360-0529; Tel: +91-80-2293-2850
First published on 11th June 2025
Abstract
Correction for ‘Hydroxy-directed iridium-catalyzed enantioselective formal β-C(sp2)–H allylic alkylation of α,β-unsaturated carbonyls’ by Sankash Mitra et al., Chem. Sci., 2022, 13, 12491–12497, https://doi.org/10.1039/D2SC03966D.
The authors regret that in the original article, the absolute configuration of 3-phenyldihydrofuranone 8 was assigned to be (R) by comparing its specific rotation with that reported in the literature.1 Accordingly, the absolute configuration of 3aa was assigned as (R) and those of the other products (3), shown in Tables 2 and 3, were inferred as the same by analogy.
However, recent literature reports2,3 revealed the absolute configuration of 8 to be (S), as shown below:
Accordingly, the absolute configuration of 3aa was reassigned as (S). As a consequence, the newly generated allylic stereocenter in all the β-allyl 3-hydroxypyranone derivatives (shown in Tables 1–3) as well as the compounds derived from them (Scheme 2) in the original article should be opposite.
The corrected structures and data for all the compounds have also been included in the revised ESI. The ESI has been updated as of 02/06/2025 to reflect all these changes.
These corrections, however, do not alter the conclusions of the original article.
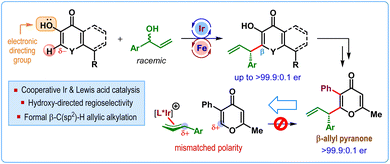
The graphical abstract image has also been updated to the following:
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370–4371 CrossRef CAS PubMed
.
- M. A. Emmanuel, N. R. Greenberg, D. G. Oblinsky and T. K. Hyster, Nature, 2016, 540, 414–417 CrossRef CAS PubMed
.
- Y. Hayashi, Y. Hatano and N. Mori, Synlett, 2022, 33, 1831–1836 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |