Open Access Article
Open Access ArticleDesign, synthesis and anticancer evaluation of novel hydrazide-2-oxindole analogues as GSK-3β kinase inhibitors†
Ashok Madarakhandi ab,
Sujeet Kumar
ab,
Sujeet Kumar c,
Nishith Teraiya
c,
Nishith Teraiya d,
Dominique Schols
d,
Dominique Schols e,
Soujanya J. Vastrad
e,
Soujanya J. Vastrad f,
P. Shyamjith
f,
P. Shyamjith g,
Bibha Choudhary
g,
Bibha Choudhary g,
Arzoo Raih and
Subhas S. Karki
g,
Arzoo Raih and
Subhas S. Karki *ab
*ab
aDepartment of Pharmaceutical Chemistry, Dr Prabhakar B. Kore Basic Science Research Center, Off-Campus, KLE College of Pharmacy, Bengaluru-560010, India. E-mail: subhasskarki@gmail.com; subhashkarki@klepharmblr.org; Fax: +91 8023425373; Tel: +91 80 23325611
bKLE Academy of Higher Education & Research, Belagavi-590010, Karnataka, India
cDepartment of Pharmaceutical Chemistry, Nitte College of Pharmaceutical Sciences (Nitte-Deemed to be University, Mangaluru), Yelahanka, Bengaluru, Karnataka 560064, India
dDepartment of Pharmaceutical Chemistry, K. B. Institute of Pharmaceutical Education and Research, Kadi Sarva Vishvavidyalaya, Gandhinagar 382023, Gujarat, India
eRega Institute for Medical Research, Department of Microbiology, Immunology and Transplantation, Laboratory of Virology and Chemotherapy, KU Leuven, B-3000 Leuven, Belgium
fDepartment of Pharmacy Practice, Faculty of Pharmacy, M.S. Ramaiah University of Applied Sciences, Bengaluru 560054, Karnataka, India
gInstitute of Bioinformatics and Biotechnology, Electronic City Phase 1, Bengaluru, India
hSchool of Applied Material Science, Central University of Gujarat, Gandhinagar 382030, Gujarat, India
First published on 14th July 2025
Abstract
GSK-3β plays an essential role in cancer progression, making it a promising target for therapeutic intervention with glycogen synthase kinase (GSK-3β) inhibitors. The designed compounds were discovered for their potential role against the proliferation of cancer via targeting GSK-3β. In the present study, hydrazide-2-oxindole analogues were designed, synthesised, and evaluated for anticancer efficacy against GSK-3β. Compounds were screened against Capan-1, HCT-116, LN-229, MCI-4460, DND-41, HL-60, K-562, MOLT4, Z-138 cells and normal cell line HEK 293. Among the compounds, 6Eb and 6Ec showed the highest selective cytotoxicity against prostate cancer (Capan-1) with CC50 values of 9.40 μM, and 8.25 μM, respectively. Furthermore, the mechanism of the anticancer effect was evidenced by 6Eb and 6Ec against GSK-3β kinase with IC50s of 11.02 μM and 59.81 μM, respectively. In addition, elevation of β-catenin and downregulation of NF-kB and STAT3 in the western blot by 6Eb evidenced inhibition of GSK-3β kinase as the cause of cytotoxicity. Furthermore, in vitro results were supported by docking scores of −10.5 kcal mol−1 and −8.8 kcal mol−1, respectively, as compared to bio-acetoxime (−7.7 kcal mol−1). Furthermore, the higher stability in molecular dynamics simulations validated the docking approach and indicated anti-cancer effects by inhibiting GSK-3β. In addition, the density functional theory analysis identified electronic distribution in compounds, which was correlated to the findings of docking and molecular dynamic simulation on their participation in polar and lipophilic interactions with kinase. Moreover, the compounds meet the drug-likeness criteria, suggesting they could be candidates for the development of drugs against cancer that target GSK-3β.
1. Introduction
Many human disorders, including cancer, have been linked to abnormal GSK-3β activity, which suggests that it could be a therapeutic target for anticancer treatment. In around 25 distinct cancer types, GSK-3β has been recognized as a possible therapeutic target in recent investigations, with many of these studies being published in the last decade. In addition, there is growing evidence that blocking GSK-3β activity shields healthy cells and tissues from the negative consequences linked to traditional cancer treatments.1–4 Although only active GSK-3β is expressed in cancer cells, abnormal nuclear accumulation of GSK-3β has been recognized as a characteristic of cancer cells in malignant tumors of various origins.5–8 Recent findings have highlighted the critical involvement of GSK-3β in the anticancer immune response.9–13GSK-3, a serine/threonine kinase, exists in two isoforms: GSK-3α and GSK-3β. Multiple processes, such as phosphorylation, protein complex formation, and subcellular distribution, contribute to GSK-3, activity regulation.14,15 So far, GSK-3β has been linked to several illnesses, including diabetes, cancer, bipolar disorder, and neurodegenerative disorders. GSK-3β regulates apoptosis, proliferation, and the cell cycle.16 In contrast; GSK-3β has been described as a tumour suppressor in malignancies such as skin, breast, oral cavity, and lung cancer. There are two types of GSK-3β inhibitors: non-ATP competitive inhibitors and ATP competitive inhibitors. Non-ATP competitive inhibitors form a weak binding association with the enzyme and do not compete with ATP concentration, providing a distinct pharmacological advantage. The bulk of known GSK-3β inhibitors are ATP-competitive, targeting the ATP-binding region of GSK-3β. As illustrated in Fig. 1, there are multiple inhibitors from various chemical classes, including bisindolylmaleimide, indolyl-maleimide, pyrimidine, maleimide, indirubine, paullone, pyrazolamide, oxadiazole, thiazole, and thiadiazole.17,18
 | ||
| Fig. 1 Pharmacophores of GSK-3β inhibitors. The key structural components involved in the interaction are highlighted. | ||
Staurosporine, a natural substance from Streptomyces staurosporeus, inhibits GSK-3β with an IC50 of 15 nM.19 Compound A is a 7-azaindazolyl-indolyl-maleimide analogue with a morpholine side chain that fills the expanded pocket via H-bonds with Lys183 and Asp200.19 The ketone and amine of indolyl-maleimide inhibitor, B, interacted via polar contacts (Fig. 1). Furthermore, the side chain of aliphatic amino alcohol stabilized the complex from the end via an H-bond with Gln185.20 The nitrogen in the pyrimidine and anilino rings of compound C was connected to Tyr134 and Val135 via H-bonds. While in the kinase's back pocket, amide and hydroxyl groups protruded and established additional hydrogen bond with Asp200. Furthermore, their oral bioavailability was comparable in rats (F = 65%; t1/2 = 3 h) and mice (F = 67%; t1/2 = 4.4 h).21 In in vivo research on zebrafish embryos, maleimide analogue D demonstrated a higher affinity and IC50 of 92 μM. An SAR investigation found that substituents at the C-4 or C-5 locations fit into the front area of the ATP site. Larger substitutions had more hydrophobic interactions and a stronger binding affinity.22 Likewise, indirubin analogue E showed higher selectivity against GSK-3β with an IC50 of 22 nM compared to other kinases. The isatin ring's amino and ketone groups created strong hydrogen bonds and lipophilic contacts to stabilize the complex.23,24 Paullone analogue F, containing a cyclic amide and a nitro group that enhanced molecular interactions with the target, demonstrated potent kinase inhibition with an IC50 of 4 nM.25 Likewise, pyrazolamide analogue G exhibited strong kinase binding through a combination of polar and nonpolar interactions, mediated by its amide linkage and phenyl ring, also resulting in an IC50 of 4 nM.26 The compound ‘H’ interacts with the ATP binding site, where the O1 oxygen atom and hydrogen atom on the C2-carbon of the benzodioxole form hydrogen bonds with the amide NH hydrogen and the carbonyl oxygen of Val135 located in the hinge region, respectively. The 4-methoxy-3-fluorobenzyl group occupies the hydrophobic pocket, while both the N3 and N4 nitrogen atoms of the oxadiazole are involved in a distinctive hydrogen bond network connecting Lys85–Glu97–Asp200 through two water molecules.27 Moreover, the urea linkage of compound I formed a key interaction with Gln137 at the centre of the binding site. The methoxyphenyl ring further stabilized the complex by occupying the rear pocket and forming close contact with Ile62, Lys183, and Gln185.28 Additionally, the thiadiazolidinone ring ketones in TDZD-8 (J) established hydrogen connections with Lys206 and Arg96.29 Pharmacophore searches revealed common pharmacophoric components such as amide and urea linkages (open and cyclic), oxime, and a fused ring involved in polar contact. In addition, the core ATP binding site was occupied by the parent heterocyclic ring, which included pyrrole, pyrazole, oxadiazole, thiazole, thiadiazole, indole, and pyrimidines (Fig. 1). Furthermore, substituent's like nitro, methoxy, bromo, fluoro, methyl, and ethyl boosted activity by interacting with the backside of the pocket. Inspired by these findings, we decided to include heteryl rings, such as triazole and indole rings, as well as the linkage of hydrazide in our new molecules to preserve similar types of interactions.
1.1. Rationale and design
Several indolylmaleimides, such as A and B, have been developed to be effective GSK-3β inhibitors based on staurosporine, a microbial alkaloid discovered as an initial GSK-3β inhibitor (Fig. 1). The majority of these indolylmaleimides exhibit toxicity, low solubility, and poor selectivity, making them inappropriate for treating disorders such as diabetes, Alzheimer's disease, and cancer.19,30 As a result, we chose smaller thiadiazolidinone synthetic analogues TDZD-8 and 10, the first non-competitive GSK-3β inhibitors that lack all of the aforementioned toxicities and exhibit drug-like characteristics. TDZD-8 and 10 inhibited GSK-3β at 2 and 10 μM, respectively. They trigger apoptosis, causing extremely rapid cell death and loss of membrane integrity.29 Furthermore, indirubin-3-monoxime analogues such as bio-acetoxime, and 44 were more selective against GSK-3β than other kinases, with affinities of 22 nM and 3 nM, respectively.31 In SY5Y-MYCN cells, bio-acetoxime significantly lowers c-MYC expression and p-SMAD3 levels. It also reduces the viability of KCN, KCNR, SY5Y, Kelly, and IMR32 cells by inducing apoptosis.32 The increased potency of these analogues prompted us to investigate them as potential leads for the development of design compounds via molecular hybridization approach. The thiadiazolidinone ring's ketones in TDZD-8 formed two hydrogen connections with Lys206 and Arg96. To keep the binding pattern consistent, we bioisosterically substituted the thiadiazolidinones with a triazole ring in our proposed molecule. Furthermore, the amino and ketone groups of the indole ring in bio-acetoxime and compound 44 formed strong H-bonds with Asp133, Val135, and Asp200. Additionally, the indole ring increased binding affinity through numerous lipophilic interactions with Ala83, Tyr134, and Leu188. Because of it's significant contribution, we bioisosterically replaced the benzyl ring of TDZD-8 in our developed compound with indole moiety to retain a similar interaction. Furthermore, the monoxime group in the bio-acetoxime stabilized protein–ligand complex by filling the back side of the pocket. To preserve the same stability and binding connection in our developed compounds, we added different substituted hydrazides to the indole ring (Fig. 2).In the present study, a series of indole-linked triazoles (6Ab–Ed) were designed, synthesized, and evaluated for their anti-proliferative activity. Their mechanism of action was elucidated through a combination of GSK-3β kinase inhibition assays, western blot analysis, and in silico studies.
2. Materials and methods
2.1. Chemistry
All chemicals and reagents were procured from BLD Pharmatech, Hyderabad, and Spectrochem Bengaluru, India. The progress of reactions was monitored by thin layer chromatography (TLC) on pre-coated silica gel 60 F254 plates (Merck, Germany) with chloroform and ethanol as the mobile phase. Melting points (MP) were determined using a digital melting point apparatus (DBK 50 Hz, 220 V) and reported uncorrected. Proton and carbon nuclear magnetic resonance (1H/13C NMR) spectra were recorded on a 400/100 MHz Bruker instrument in CDCl3/DMSO-d6 and chemical shift values (J) were reported in hertz (Hz). Fourier-transform infrared (FT-IR) spectra were obtained on JASCO460+ FTIR spectrophotometer. High-resolution mass spectrometry (HRMS) data were collected by electrospray ionization (ESI) technique using Waters Synapt G2 QTOF mass spectrometry. The 1-(prop-2-yn-1-yl)indoline-2,3-dione (3) was synthesized by reacting indole-2,3-dione (1) with 3-bromoprop-1-yne (2) in presence of K2CO3 in dimethylformamide (DMF).33 The respective aryl azides (4A–E) were obtained by reacting aryl bromides with sodium azide as per literature.33 Other intermediate derivatives (5A–D) were prepared according to literature (Scheme 1).34 | ||
| Scheme 1 Synthesis of 3-substituted-1-((1-benzyl-1H-1,2,3-triazol-4-yl) methyl)indoline-2-one (6Ab–Ed). | ||
2.2. General procedure for synthesis of 1-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-3-hydrazonoindolin-2-one (6A–E)
1-((1-Benzyl-1H-1,2,3-triazole-4-yl)methyl)indoline-2,3-dione (5A–E) 1.0 mmol, and respective hydrazines (R1NHNH2) were refluxed in 50 mL absolute ethanol and 0.5 mL glacial acetic acid. The reaction mixture was cooled and, the precipitated mass of 6 was re-crystallized from the ethanol-chloroform mixture in appropriate ratio.2.3. Biological investigation
Suspension cell lines were seeded at densities ranging from 2500 to 5000 cells per well in 384-well culture plates containing the test compounds at the same concentration points. All cell lines were incubated for 72 h with compounds and then analyzed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) reagent (Promega, Leiden, The Netherlands) according to the manufacturer's instructions. Absorbance of the samples was measured at 490 nm using a SpectraMaxPlus 384 (Molecular Devices, CA, USA), and OD values were used to calculate the 50% inhibitory concentration (IC50). Compounds were tested in two independent experiments.35
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, NF-kB (BioLegend) at 1
5000, NF-kB (BioLegend) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, PTEN (CST) at 1
1000, PTEN (CST) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, STAT-3 (CST) at 1
1000, STAT-3 (CST) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, β-catenin at 1
1000, β-catenin at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. Anti-rabbit IgG-HRP (CST) at 1
1000. Anti-rabbit IgG-HRP (CST) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, and anti-mouse-IgG-HRP (CST) at 1
1000, and anti-mouse-IgG-HRP (CST) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 and 1
5000 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. Protein bands were visualised using the enhanced chemiluminescence substrate from Bio-Rad. Images were captured on the Bio-Rad Doc.
1000. Protein bands were visualised using the enhanced chemiluminescence substrate from Bio-Rad. Images were captured on the Bio-Rad Doc.2.4. Computational (in silico) study
| ΔGbind = Gcomplex − (Gprotein + Gligand) | (1) |
3. Results and discussion
3.1. Chemistry
A total of fifteen indole linked 1,2,3-triazoles (6Ab–Ed) were prepared by reacting 1.0 mmol of 1-((1-benzyl-1H-1,2,3-triazole-4-yl)methyl)indoline-2,3-dione (5A–E), and respective hydrazines (R1NHNH2). All structures were confirmed for their structure by FTIR, 1H/13C NMR and, HRMS data. In the FT-IR spectra of synthesized derivatives (6Ab–6Ed), the indole-NH stretching peaks appeared between 3477–3130 cm−1 while, the aromatic and aliphatic –CH stretching were observed in the range of 3210–3021 and 2999–2841 cm−1 respectively. Intense peaks for carbonyl (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching appeared between 1692–1661 cm−1. In 1H-NMR spectra of 6Ab–Ed the –NH protons appeared between δ 13.88–12.26 ppm. Aromatic protons observed in the range of δ 9.38–6.84 ppm. Peaks for –CH2 protons appeared between δ 5.75–5.01 ppm. The methoxy protons (–OCH3) of 6Eb, 6Ec & 6Ed appeared at δ 3.72 ppm. The methyl protons (–CH3) of 6Cb, 6Cc & 6Cd appeared at δ 2.26 ppm. The 13C NMR spectra of all molecules had shown peak for C
O) stretching appeared between 1692–1661 cm−1. In 1H-NMR spectra of 6Ab–Ed the –NH protons appeared between δ 13.88–12.26 ppm. Aromatic protons observed in the range of δ 9.38–6.84 ppm. Peaks for –CH2 protons appeared between δ 5.75–5.01 ppm. The methoxy protons (–OCH3) of 6Eb, 6Ec & 6Ed appeared at δ 3.72 ppm. The methyl protons (–CH3) of 6Cb, 6Cc & 6Cd appeared at δ 2.26 ppm. The 13C NMR spectra of all molecules had shown peak for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S at δ 179 ppm, between δ 162.89 to 160.88 ppm for C
S at δ 179 ppm, between δ 162.89 to 160.88 ppm for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and between δ 159.60 to 109.83 ppm for aromatic carbons, at δ 55.89 ppm for OCH3 and between δ 53.33 to 34.77 ppm for –N–CH2, at δ 21.13 ppm for CH3 carbons. The m/z values of all molecules (6Ab–Ed) were found in close proximity with calculated mass of the molecule and confirming their structure.
O, and between δ 159.60 to 109.83 ppm for aromatic carbons, at δ 55.89 ppm for OCH3 and between δ 53.33 to 34.77 ppm for –N–CH2, at δ 21.13 ppm for CH3 carbons. The m/z values of all molecules (6Ab–Ed) were found in close proximity with calculated mass of the molecule and confirming their structure.
3.2. Cytotoxicity study
The cytotoxicity studies in a series of hydrazide-2-oxindole analogues (6Ab–Ed) carried out across a broad range of human cancer cell lines include Pancreatic adenocarcinoma (Capan-1), Colorectal carcinoma (HCT-116), Glioblastoma (LN229), Lung carcinoma (NCI-H460), Acute lymphoblastic leukaemia (DND-41), Acute myeloid leukaemia (HI-60), Chronic myeloid leukaemia (K562), T lymphoblast (MOLT4), Non-Hodgkin lymphoma (Z138) and normal human embryonic kidney (HEK 293) cell lines. Sunitinib and 5-Fluorouracil were used as reference standard during the study. In comparison to sunitinib (1.5 μM) and 5-fluorouracil (2.7 μM), molecules 6Eb and 6Ec exhibited moderate cytotoxicity specifically against Capan-1 cells with IC50 values in the range of 8.25–9.40 μM indicating some level of cytotoxicity over other molecules in the series. Two more compounds namely 6Cb and 6Eb showed cytotoxicity against LN229 cells (CC50: 10.20–12.55 μM). The compounds 6Eb and 6Ec displayed promising toxicity against MOLT4 cells with 2.7 and 0.9 μM respectively (ESI Fig. 1†). Rest of the molecules failed to exhibit significant cytotoxicity against tested human cancer cell lines. To test these compounds toxicity on normal cells, both 6Eb and 6Ec were tested on HEK 293 cells and found to be non-toxic with IC50 46 μM and 144 μM respectively. These results indicate that these two molecules are non-toxic to normal cells with the great selectivity to cancer cells. The study suggests that the substituent on the triazole benzyl ring (specifically –H or electron donating CH3 and 4-OCH3) and the carbothiamide and pridazinone substitutions on the hydrazide nitrogen are critical in the observed cytotoxicity by 6Cb, 6Eb and 6Ec. Based on cytotoxicity data we can conclude that specific substituents other than –H or methyl and methoxy became potent cytotoxic compounds, particularly against the Capan-1 and LN229 cells. Further, despite the moderate activity of 6Cb, 6Eb against LN229, & 6Eb, 6Ec against Capan-1 cells, none of the derivatives exhibited notable cytotoxicity against other cancer cell lines suggesting, a certain degree of selectivity for LN229 and Capan-1 cells. Overall these compounds are selectively potent against adherent type cancer cell lines tested but not to suspension type cancer cell lines (Table 1).| Compounds | Cytotoxicity adherent cells (CC50) 100 μM | Cytotoxicity suspension cells (CC50) 100 μM | ||||||
|---|---|---|---|---|---|---|---|---|
| Capan-1 | HCT-116 | LN229 | NCI-H460 | DND-41 | Hl-60 | K562 | Z138 | |
| 6Ab | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Ac | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Ad | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Bb | >100 | 54.05 ± 0.71 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Bc | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Bd | 74.30 ± 0.28 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Cb | 41.95 ± 2.33 | 70.70 ± 3.25 | 12.55 ± 0.78 | >100 | >100 | >100 | >100 | >100 |
| 6Cc | 62.15 ± 7.28 | >100 | 70.85 ± 10.96 | >100 | >100 | >100 | >100 | >100 |
| 6Cd | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Db | >100 | >100 | 23.55 ± 1.91 | >100 | >100 | >100 | >100 | >100 |
| 6Dc | 26.10 ± 3.82 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Dd | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 6Eb | 9.40 ± 0.57 | 46.30 ± 6.22 | 10.20 ± 0.99 | >100 | >100 | >100 | >100 | >100 |
| 6Ec | 8.25 ± 0.78 | 34.25 ± 5.16 | 67.90 ± 1.70 | >100 | 73.80 ± 14.42 | 76.05 ± 3.75 | 88.85 ± 10.25 | 53.80 ± 2.12 |
| 6Ed | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Sunitinib | 1.5 ± 0.01 | 4.25 ± 0.71 | 11.80 ± 1.41 | 8.85 ± 3.04 | 12.90 ± 1.98 | 5.00 ± 1.27 | 8.4 ± 2.12 | 8.15 ± 1.49 |
| 5-Fluorouracil | 2.7 ± 0.01 | 2.3 ± 0.71 | 7.35 ± 0.35 | 5.00 ± 1.70 | 65.35 ± 4.60 | 5.90 ± 0.99 | 12.90 ± 0.42 | 7.85 ± 0.71 |
3.3. Inhibition study of GSK-3β kinase
A kinase activity experiment was conducted in the presence of inhibitors to determine their specificity for GSK-3β. A reaction mixture containing 50 μM ATP, 50 μM DTT, 1 μg per μL GSK-3β substrate, and 0.5 ng per μL GSK3β enzyme, together with increasing quantities of the 6Eb and 6Ec (1 μM to 125 μM), was incubated. The reaction mixture including DMSO served as the vehicle control, whereas 12 nM laduviglusib was employed as a positive control in the experiment. At sub-micromolar concentrations, GSK-3β inhibitors reduced their efficacy by 50%. Specifically, 6Eb exhibited 50% inhibition of activity at a concentration of 11.02 ± 1.95 μM. The activity of 6Eb was 60%, 50%, and 40% at concentrations of 5 μM, 7.5 μM, and 10 μM, respectively. These observations suggest that 6Eb decreased the activity of GSK-3β in a concentration-dependent manner. While compound 6Ec showed a 50% inhibition at higher values of 59.81 ± 7.56 μM (Fig. 3A–C). These investigations suggest that our designed compounds acted as anticancer agents via inhibition of GSK-3β.3.4. 6Eb regulated GSK-3β targets NF-kB and β-catenin
To check the activity of the GSK-3β inhibitors in cells, MOLT4 cells were treated with varying concentrations of 6Eb and 6Ec and the IC50 was calculated based on MTT assay (ESI Fig. 1a and b†). In MOLT4 cells, IC50 for 6Eb and 6Ec was 2.7 and 0.9 μM respectively, which was lowest among all the cell lines screened for GSK-3β inhibiton. We performed western blot for select GSK-3β targets, NFkB, β-catenin and PTEN. 6Eb showed downregulation of NF-kB, upregulation of β-catenin and no change in PTEN levels. It is very well known that phosphorylation of β-catenin by GSK-3β leads to ubiquitin mediated destruction of β-catenin, therefore inhibition led to an increase in β-catenin. Upregulation of β-catenin and downregulation of NF-kB lead to increase in cytotoxicity upon GSK-3β inhibiton. 6Ec did not show any change in the levels of any of the proteins tested (Fig. 4). To check whether 6Ec downregulated any of the GSK-3β target, we selected STAT3 (ESI Fig. 2a and b†), and found downregulation of STAT3 in 6Eb and not 6Ec, which is also evident from the kinase assay suggesting low kinase inhibitor activity of 6Ec, although higher cytotoxicity was observed with 6Ec.3.5. In silico study
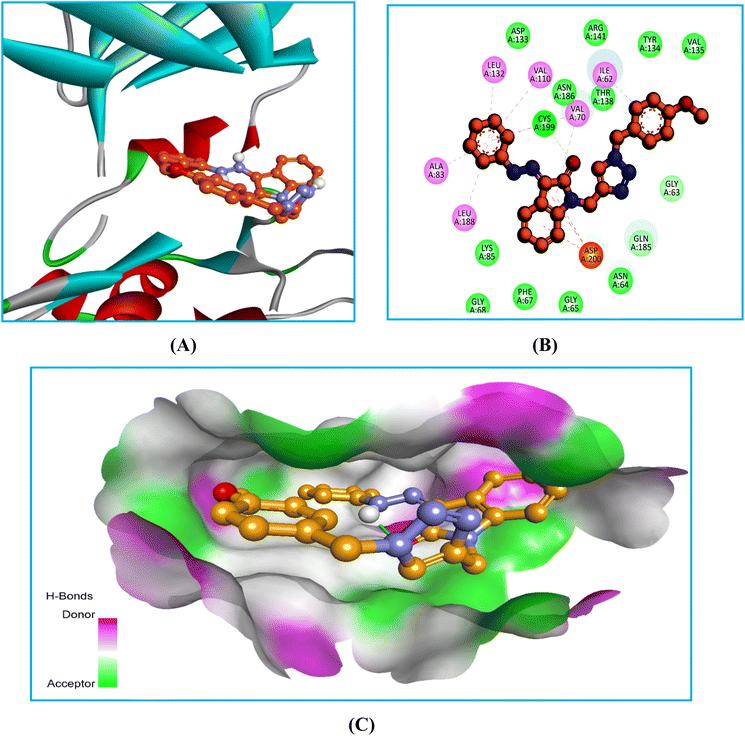
There were strong lipophilic interactions between the phenylhydrazone ring of compound 6Ec and Ala83, Val110, Leu132, and Leu188 at the end of the kinase. These numerous interactions keep the molecule in a stable shape and stabilize the ligand–protein combination. A p-methoxyphenyl ring connected the kinase with Ile62 via lipophilic contacts at its extremities. The ketone of the indole ring also improved the stability of the complex through H-bonds and lipophilic interactions with Cya199 and Val70. Furthermore, the indole ring increased binding affinity by supporting the kinase from the center via a significant multiple pi-cation interaction with Asp200. The binding and stability of the protein–ligand complex to GSK-3β were significantly influenced by many interactions, including pi-cation interactions, lipophilic contact, and H-bonds. These findings align with their biological investigation, indicating that compound 6Ec exhibited anticancer effects through GSK3β inhibition.
The replacement of a smaller linkage of hydrazinecarbothioamide with phenylhydrazide in 6Ec changed the orientation within the pocket, but it retained binding with comparable residues. These changes in 6Ec could be attributed to the presence of two bulky phenyl rings, such as p-methoxyphenyl and phenylhydrazide, on both sides of the molecule. In contrast, 6Eb had an aliphatic part of hydrazinecarbothioamide on one side and p-methoxypheyl on the other. The change in the substitution pattern causes both compounds to orientate differently within the same pocket occupied by standard.
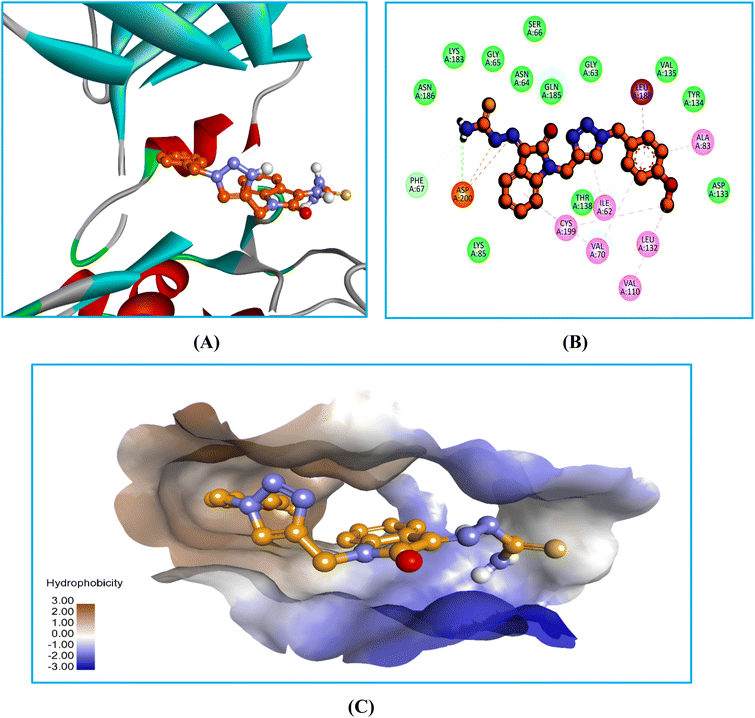
In the bio-acetoxime, the ketone group at the second position of indole formed strong hydrogen bonds with Lys85 and Asp200. The presence of bromine at the C6 position of indole increased lipophilic interactions with Ala83, Tyr134, and Leu188. Furthermore, indole showed many lipophilic and pi-cation interactions with Val70 and Leu188 and Cys199, respectively. Furthermore, strong pi-cation interactions between the indole ring and Asp200 were observed, stabilizing the protein–ligand complex from one end. The images in (Fig. 5–7) demonstrated that our developed compounds bind to residues and binding sites similar to those of the standard. Moreover, their higher docking score indicates that these compounds could act as an anticancer agent by targeting GSK-3β.
Root Mean Square Deviation (RMSD) is a measure of how far atoms move from one frame to another. During simulation, the RMSD of the protein and ligand can reveal structural conformation and ligand stability in relation to the protein and binding site. Changes of 1–3 Å are suitable for small and globular proteins.37 Compound 6Ec and protein stabilized after modest alterations over the first 15 ns of MD simulation. After a brief period of drifting (17–19 ns), it returned and remained steady for the following 31 ns. Between 32–37 ns and 40–41 ns, there was another drift away from the complex with minor fluctuations. After 43 ns, it stayed connected to the protein for the remainder of the simulation session. The RMSD value for compound 6Ec and GSK-3β was 1 Å, which falls within the acceptable range of 1–3 Å, demonstrating stability (Fig. 8A and B).
The RMSD plot of the target GSK-3β with the bound ligand bio-acetoxime (standard) in (Fig. 8B) demonstrated that the ligand–protein complex was stable within the first 5 ns, with a minor fluctuation at a distance of 3.0 Å. Despite moving away from the protein for 37–68 ns, the ligand returned to its original position and remained fixed until the simulation was over. The RMSD value for target GSK-3β with standard was around 1.0 Å, which is within acceptable limits (1–3 Å).
| Compound | ΔGbind | ΔGbindLipo | ΔGbindvdW | ΔGbindCoulomb | ΔGbindHbond | ΔGbindSolvGB | ΔGbindCovalent |
|---|---|---|---|---|---|---|---|
| 6Ec | −66.019 | −27.785 | −59.977 | −12.463 | −1.433 | 14.968 | −4.068 |
| Bio-acetoxime | −49.204 | −15.185 | −45.547 | −22.258 | −1.572 | 13.730 | −2.151 |
The lowest energy of optimization in the ground suggests the most stable conformation of 6Ec (obtained using the B3LYP/6-31G(d) level of theory). It had a higher dipole moment implies a greater potential to undergo polar interaction with the target protein, which was justified via pi-cation and H-bond interaction in the docking (Fig. 10 and Table 3).
 | ||
| Fig. 10 Ground state optimized and labeled structures of 6Ec were calculated using DFT/B3LYP/6-31G(d) level of theory. | ||
| Compound | Optimisation energy (hartree) | Dipole moment (debye) |
|---|---|---|
| 5Ec | −1444.826 | 3.709 |
In order to understand molecular properties of a neutral system, we calculated the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). The energy gap between EHOMO and ELUMO was found to be 0.12559 eV, which was less than 0.2 eV, indicating significant chemical reactivity and less kinetic stability of the molecules (Fig. 11). These findings were also supported by higher softness and lower hardness values. Together, these might reflect that 6Ec had a higher affinity for the target protein. Furthermore, lower values of ionization potential and chemical potential showed that the compound had a considerable inclination to accommodate the ionic state as well as greater reactivity towards the target (Table 4), which has verified the finding of approximately six types of total interaction in the MD investigation. Furthermore, increased reactivity of 6Ec was observed in docking studies via multiple types of lipophilic, pi-cation, and H-bond interactions. Furthermore, lower values of electronegativity, electron affinity, and electrophilicity index may suggest that 6Ec had a less electron-loving nature, which could be due to the presence of an electron-donating group-containing ring such as p-methoxyphenyl, as well as basic hydrazone linkage and a triazole ring. Furthermore, HOMO images revealed a greater electron density on the indole to phenylhydrazone area. In contrast, the indole ring had a higher concentration of LUMO than the phenylhydazone ring, which could be attributed to the presence of an electron-withdrawing carbonyl group. It resulted in a higher electron deficit in LUMO, which may be cause of one lipophilic contact with Val70 during the docking experiment.
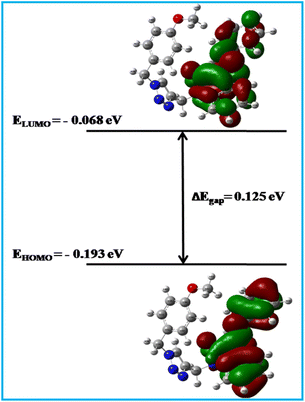 | ||
| Fig. 11 Frontier molecular orbitals (HOMO and LUMO) and their energy gap (ΔEgap) of 6Ec were calculated using the DFT/B3LYP/6-31G method. | ||
| EHOMOa (eV) | ELUMOb (eV) | ΔEgapc (eV) | Id (eV) | Ae (eV) | μf (eV) | χg (eV) | ηh (eV) | Si (eV) | ωj (eV) |
|---|---|---|---|---|---|---|---|---|---|
| a Energy of HOMO.b Energy of LUMO.c Energy gap between HOMO & LUMO.d Ionization potential.e Electron affinity.f Chemical potential.g Electronegativity.h Hardness.i Softness.j Electrophilicity index. | |||||||||
| −0.19345 | −0.06786 | 0.12559 | 0.19345 | 0.06786 | −0.130655 | 0.130655 | 0.062795 | 7.962417 | 0.135975 |
Also, the molecular electrostatic potential (MEP) map tells about reactivity regions in an organic molecule depending upon the electron density of each atom, which is the most probable region for electrophilic attack. Here, different colors represent different values of electrostatic potential, molecular size, and shape. Potential increases in order from red < orange < yellow < green < blue.39–41 High electron density is manifested by the red zone towards the triazole ring, hydrazone linkage, and oxygen of indole in the MEP map (Fig. 12). On the other hand, a phenylhydrazone ring oriented towards yellow color indicates a slightly electron-rich region, which may be due to the electron-releasing property of the hydrazone ring. These observations also supported multiple lipophilic and polar interactions of phenylhydrazone with Ala83, Val110, Leu132, Leu188, Cys199, and Asp200 in the docking and MD study. Taken together, the promising electrical and molecular features of 6Ec warrant further biological investigation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), topological polar surface area (tPSA), and Lipinski's rule of five (Ro5) were computed using the Swiss-ADME web server.
P), topological polar surface area (tPSA), and Lipinski's rule of five (Ro5) were computed using the Swiss-ADME web server.In silico results summarized in (Table 5) reveal that compound 6Ec had a greater drug-likeness property over 6Eb and standard among the studies. 6Ec exhibited a tPSA value (84.64 Å2) within the range of ≤140, which is ideal for improved oral absorption and intestinal permeability. Both compounds showed H-bonds within the range, indicating that aqueous solubility and binding affinity were optimized without compromising solubility. Both compounds 6Eb and 6Ec had excellent log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (2.96 and 1.98), indicating sufficient lipophilicity to permeate cellular membranes without CNS damage. Further, 6Eb and 6Ec had optimum number of rotatable bonds, which may led to greater rigidity, higher binding affinity, and lower conformational changes during target engagement. Besides, Fsp3 indicates a saturation of sp3 hybridized carbons. A greater score of Fsp3 indicates a drug's similarity with known medicines, which often contain a larger proportion of saturated carbon atoms. Also, drug–drug interactions, metabolic interactions, and toxicity typically decrease with increasing Fsp3 values. While, 6Ec exhibited a lower Fsp3 value compared to bio-acetoxime (standard), suggesting a more rigid interaction with the target. The finding collectively reflects that 6Ec had a better ADME profile than 6Eb and bio-acetoxime (standard). Moreover, it demonstrated a balanced set of properties favouring optimal absorption, distribution, and target binding. Additionally, it met the necessary tPSA, lipophilicity, Fsp3, and binding affinity. These characteristics collectively suggest that 6Ec could be a promising candidate with better pharmacokinetics and smaller metabolic liabilities.
P values (2.96 and 1.98), indicating sufficient lipophilicity to permeate cellular membranes without CNS damage. Further, 6Eb and 6Ec had optimum number of rotatable bonds, which may led to greater rigidity, higher binding affinity, and lower conformational changes during target engagement. Besides, Fsp3 indicates a saturation of sp3 hybridized carbons. A greater score of Fsp3 indicates a drug's similarity with known medicines, which often contain a larger proportion of saturated carbon atoms. Also, drug–drug interactions, metabolic interactions, and toxicity typically decrease with increasing Fsp3 values. While, 6Ec exhibited a lower Fsp3 value compared to bio-acetoxime (standard), suggesting a more rigid interaction with the target. The finding collectively reflects that 6Ec had a better ADME profile than 6Eb and bio-acetoxime (standard). Moreover, it demonstrated a balanced set of properties favouring optimal absorption, distribution, and target binding. Additionally, it met the necessary tPSA, lipophilicity, Fsp3, and binding affinity. These characteristics collectively suggest that 6Ec could be a promising candidate with better pharmacokinetics and smaller metabolic liabilities.
| Compound code | Binding affinity | MW | Fsp3 | RB | HBA | HBD | tPSA | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Lipinski | ESOL log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S |
ESOL class | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KP KP |
F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6Eb | −8.8 | 421.48 | 0.15 | 7 | 5 | 2 | 142.75 | 1.98 | 0 | −3.75 | Soluble | −7.36 | 0.55 |
| 6Ec | −10.1 | 438.48 | 0.12 | 7 | 5 | 1 | 84.64 | 2.96 | 0 | −5.08 | Moderately soluble | −6.19 | 0.55 |
| Bio-acetoxime | −7.7 | 73.09 | 0.67 | 0 | 2 | 1 | 32.59 | 1.22 | 0 | −0.37 | Very soluble | −6.66 | 0.55 |
4. Conclusions
The current work examines the cytotoxic effects of newly synthesized hydrazide-2-oxindole analogues against several cancer cell lines. The compound 6Cb exhibited cytotoxicity against the LN229 cell line (CC50: 12.22 μM). The other two compounds, 6Eb and 6Ec, demonstrated the maximum cytotoxicity against the MOLT4 cells (2.7 μM and 0.9 μM) followed by Capan-1 cell line (CC50: 9.40 μM and 8.25 μM, respectively). These compounds 6Eb and 6Ec were non-toxic to normal cells (HEK 293 IC50 46 μM and 144 μM respectively). Furthermore, the compounds 6Eb and 6Ec strongly inhibited GSK-3β, indicating an anticancer mechanism. In western blot, 6Eb enhanced the β-catenin fraction and decreased NF-kB and STAT3 expression, confirming its anticancer mechanism. Also, docking and MD tests corroborated cytotoxicity, kinase inhibition, and western blotting results, demonstrating that compound 6Eb inhibited GSK-3β activity. Moreover, DFT analysis revealed electron-rich and deficient regions on molecules, which were used to compare non-polar and polar interactions with kinase in docking and MD studies. Likewise, in silico ADMET discovered the drug-like properties of the proposed molecule, demonstrating its novelty as a potential lead. However, the study findings indicate that additional in vivo and biochemical studies are required to evaluate the efficacy and safety of 6Eb and 6Ec, strengthening their potential as therapeutic agents.Abbreviations
| p-SMAD3 | Mothers against decape ntaplegic homolog 3 |
| c-Myc | Cellular Myc |
| DTT | Dithiothreitol |
| PDBQT | Protein data bank, partial charge, and atom type |
| OPLS | Optimised potential for liquid simulations |
| TIP3P | Transferable intermolecular potential with three point |
| MM-GBSA | Molecular mechanics/generalised born surface area |
| NF-kB | Nuclear factor kappa-light enhancer of activated B cells |
| PTEN | Phosphatase and tensin homolog |
| STAT3 | Signal transducer and activation of transcription 3 |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuations |
| ΔGbind | Binding free energy |
| ΔGbindLipo | Lipophilic contribution to the overall binding free energy of a ligand–protein complex |
| ΔGbindvdW | van der Waals force contribution to the overall binding free energy of a ligand–protein complexs |
| ΔGbindCoulomb | Change in Gibbs free energy of binding that is specifically attributed to electrostatic interactions (coulomb interactions) between a ligand and a protein or receptor |
| ΔGbindHbond | Binding free energy of hydrogen bonds |
| ΔGbindSolvGB | Binding free energy of solvation |
| ΔGbindCovalent | Binding free energy of covalent bond |
| MEP | Molecular electrostatic potential |
| ADME | Absorption, distribution, metabolism and elimination |
Data availability
The data are included within the article and in ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks to Saumya K. Patel and Nandan Dixit from the Department of Botany, bioinformatics and climate change impacts management, School of Science, Gujarat University, Ahmedabad, Gujarat, India, for providing the MD simulation study.References
- N. Embi, D. B. Rylatt and P. Cohen, Eur. J. Biochem., 1980, 107, 519–527 CrossRef CAS PubMed.
- A. J. Harwood, Cell, 2001, 105, 821–824 CrossRef CAS PubMed.
- H. Yao, P. Shaw, C. Wrong and D. C. Wan, J. Chem. Neuroanat., 2002, 23, 291–297 CrossRef CAS.
- J. R. Woodgett, EMBO J., 1990, 9, 2431–2438 CrossRef CAS.
- H. Eldar-Finkelman, Trends Mol. Med., 2002, 8, 126–132 CrossRef CAS PubMed.
- B. W. Doble and J. R. Woodgett, J. Cell Sci., 2003, 116, 1175–1186 CrossRef CAS PubMed.
- R. S. Jope, C. J. Yuskaitis and E. Beurel, Neurochem. Res., 2007, 32, 577–595 CrossRef CAS.
- S. Frame and P. Cohen, Biochem. J., 2001, 359, 1–16 CrossRef CAS PubMed.
- L. Kim and A. R. Kimmel, Curr. Opin. Genet. Dev., 2000, 10, 508–514 CrossRef CAS.
- A. S. Wagman and J. M. Nuss, Curr. Pharm. Des., 2001, 7, 417–450 CrossRef CAS.
- P. Cohen, Eur. J. Biochem., 2001, 268, 5001–5010 CrossRef CAS PubMed.
- C. Sasaki, T. Hayashi, W. R. Zhang, H. Warita, Y. Manabe, K. Sakai and K. Abe, Neurol. Res., 2001, 23, 588–592 CrossRef CAS PubMed.
- A. Castro and A. Martinez, Expert Opin. Ther. Pat., 2000, 10, 1519–1527 CrossRef CAS.
- J. A. Bertrand, S. Thieffine, A. Vulpetti, C. Cristiani, B. Valsasina, S. Knapp, H. M. Kalisz and M. Flocco, J. Mol. Biol., 2003, 333, 393–407 CrossRef CAS PubMed.
- I. Hers, J. M. Tavare and R. M. Denton, FEBS Lett., 1999, 460, 433–436 CrossRef CAS PubMed.
- H.-C. Zhang, K. B. White, H. Ye, D. F. McComsey, C. K. Derian, M. F. Addo, P. Andrade- Gordon, A. J. Eckardt, B. R. Conway, L. Westover, J. Z. Xu, R. Look, K. T. Demarest, S. Emanuel and B. E. Maryanoff, Bioorg. Med. Chem. Lett., 2003, 13, 3049–3053 CrossRef CAS PubMed.
- E. erHaar, J. T. Coll, D. A. Austen, H. M. Hsiao, L. Swenson and J. Jain, Nat. Struct. Biol., 2001, 8, 593–596 CrossRef.
- R. Dajani, E. Fraser, S. M. Roe, N. Young, V. Good, T. C. Dale and L. H. Pearl, Cell, 2001, 105, 721–732 CrossRef CAS PubMed.
- Q. Ye, Y. Shen, Y. Zhou, D. Lv, J. Gao, J. Li and Y. Hu, Eur. J. Med. Chem., 2013, 68, 361–371 CrossRef CAS.
- L. Gong, D. Hirschfeld and Y. C. Tan, et al., Bioorg. Med. Chem. Lett., 2010, 20, 1693–1696 CrossRef CAS.
- A. M. Aronov, T. Qing and G. Martinez-Botella, et al., J. Med. Chem., 2009, 52, 6362–6368 CrossRef CAS PubMed.
- H. Zou, L. Zhou and Y. Li, et al., J. Med. Chem., 2010, 53, 994–1003 CrossRef CAS PubMed.
- L. Meijer, A. L. Skaltsounis and P. Magiatis, et al., Chem. Biol., 2003, 10, 1255–1266 CrossRef CAS.
- P. Polychronopoulos, P. Magiatis and A. L. Skaltsounis, et al., J. Med. Chem., 2004, 47, 935–946 CrossRef CAS.
- J. A. Bertrand, S. Thieffine and A. Vulpetti, et al., J. Mol. Biol., 2003, 333, 393–407 CrossRef CAS.
- J. Witherington, V. Bordas and D. Haigh, et al., Bioorg. Med. Chem. Lett., 2003, 13, 1581–1584 CrossRef CAS PubMed.
- M. Saitoh, J. Kunitomo and E. Kimura, et al., Bioorg. Med. Chem., 2009, 17, 2017–2029 CrossRef CAS PubMed.
- M. L. Selenica, H. S. Jensen and A. K. Larsen, et al., Br. J. Pharmacol., 2007, 152, 959–979 CrossRef CAS.
- A. Martinez, M. Alonso, A. Castro, C. Pérez and F. J. Moreno, J. Med. Chem., 2002, 45, 1292–1299 CrossRef CAS PubMed.
- T. Kramer, B. Schmidt and F. Lo Monte, Int. J. Alzheimers Dis., 2012, 2012, 381029 Search PubMed.
- P. Polychronopoulos, P. Magiatis, A. L. Skaltsounis, V. Myrianthopoulos, E. Mikros, A. Tarricone, A. Musacchio, S. M. Roe, L. Pearl, M. Leost, P. Greengard and L. Meijer, J. Med. Chem., 2004, 47, 935–946 CrossRef CAS.
- K. Vougogiannopoulou, Y. Ferandin and K. Bettayeb, et al., J. Med. Chem., 2008, 51, 6421–6431 CrossRef CAS.
- A. Das, G. Greco, S. Kumar, E. Catanzaro, R. Morigi, A. Locatelli, D. Schols, H. Alici, H. Tahtaci, F. Ravindran, C. Fimognari and S. S. Karki, Comput. Biol. Chem., 2023, 97, 107641 CrossRef.
- K. Aman, K. Lal, M. Murtaza, S. Jaglan, Y. Rohila, P. Singh, M. B. Singh and K. Kumari, J. Biomol. Struct. Dyn., 2024, 42, 9919–9938 CrossRef.
- T. V. de Walle, A. Theppawong, C. Grootaert, S. D. Jonghe, L. Persoons, D. Daelemans, K. V. Hecke, J. V. Camp and M. D’hooghe, Monatsh. Chem., 2019, 150, 2045–2051 CrossRef.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CrossRef CAS.
- D. Yevale, N. Teraiya, T. Lalwani, M. Dalasaniya, K. Kapadiya, R. K. Ameta, C. B. Sangani and Y. T. Duan, Bioorg. Chem., 2024, 147, 107323 CrossRef CAS PubMed.
- S. Rajamanikandan, J. Jeyakanthan and P. Srinivasan, Appl. Biochem. Biotechnol., 2017, 181, 192–218 CrossRef CAS.
- K. K. Bharadwaj, T. Sarkar, A. Ghosh, D. Baishya, B. Rabha, M. Panda, B. R. Nelson, A. John, H. I. Sheikh, B. P. Dash, H. A. Edinur and S. Pati, ChemRxiv, 2021, 193, 3371–3394 CrossRef CAS PubMed.
- L. Piao, Z. Chen, Q. Li, R. Liu, W. Song, R. Kong and S. Chang, Int. J. Mol. Sci., 2019, 20, 224 CrossRef PubMed.
- M. Kawad, S. Sangani, J. K. Parmar, R. C. Dabhi, P. S. Arya, N. Teraiya, S. Ahmed and R. K. Ameta, Polyhedron, 2024, 264, 117242 CrossRef CAS.
- A. Diana, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef.
- D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01063b |
| This journal is © The Royal Society of Chemistry 2025 |