Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lignin-based vitrimers: valorization and utilization of lignin in high-value applications
Peter K.
Karoki
 a,
Shuyang
Zhang
a,
Shuyang
Zhang
 a,
Yunqiao
Pu
a,
Yunqiao
Pu
 c and
Arthur J.
Ragauskas
c and
Arthur J.
Ragauskas
 *abc
*abc
aDepartment of Chemical & Biomolecular Engineering, University of Tennessee Knoxville, Knoxville, TN 37996, USA. E-mail: aragausk@utk.edu
bCenter for Renewable Carbon, The University of Tennessee Knoxville, Institute of Agriculture, Knoxville, TN 37996, USA
cJoint Institute for Biological Sciences, Biosciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
First published on 16th July 2024
Abstract
The research efforts directed towards addressing the environmental issues and non-recyclability of conventional plastics has triggered great attention for vitrimers as a new type of renewable plastic. Vitrimers are characterized by dynamic covalent linkages that impart strong chemical resistance and strength similar to that of thermosets, while enabling reprocessability under specific conditions. The past decade has witnessed the development of various vitrimeric chemistries that are applicable to a broad range of bio-based prepolymers with application in vitrimer synthesis. This review aims to provide an overview of the recently reported lignin-based vitrimers, laying emphasis on their thermal–mechanical properties, potential applications based on their properties, and their dynamic bond exchange mechanisms. Furthermore, important features of bio-based vitrimers such as self-healing, shape memory, renewability, and reprocessability will be discussed. Some key modifications of lignin, which are necessary to improve its compatibility with other materials and targeted vitrimeric chemistry, will also be highlighted.
1. Introduction
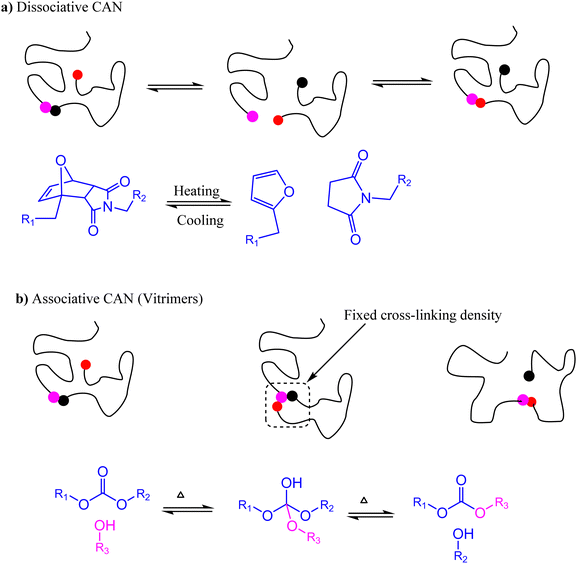
Since their discovery in 1907, synthetic polymers have been continuously engineered to become essential materials for the 20th century whose diverse properties depend on their hierarchy scales from the monomeric building blocks to the condensed matter structures. Based on internal interactions, polymers are classified into thermoplastics and thermosets. Thermoplastics are composed of linear chains that interact with each other through weak intermolecular forces and have entanglements that could be reformed at elevated temperature making thermal recycling feasible. On the other hand, thermosets have crosslinked networks with irreversible covalent bonds, which impart unique properties such as thermal stability, strong mechanical properties, and chemical resistance.1Excellent thermal–mechanical properties place thermosets as the material of choice in numerous applications such as in household appliances and electrical insulation.2 However, thermosets cannot be readily reformed or recycled due to their covalently crosslinked networks after curing,3 making eradication from the environment after use a major challenge of the 21st century. Introduction of exchangeable covalent bonds is a chemical approach used to develop dynamic cross-links, thereby imparting reprocessability of the crosslinked network.3,4 Under specific conditions, these covalent linkages detach and rebond reversibly while maintaining the structure and mechanical properties of the material. Polymers that exhibit such dynamic behavior are referred to as covalent adaptable networks (CANs).5
CANs are classified as either dissociative or associative depending on the detach–rebond mechanism of the reversible bonds. Dissociative CANs are analogous to SN1 reactions in which bond formation is preceded by bond dissociation, while associative CANs result from reversible reactions in which the original cross-links dissociate only after the new chemical cross-links are formed (Fig. 1). A good example of a dissociative CAN is a polymer network cross-linked by an adduct resulting from a mildly exothermic Diels–Alder reaction between maleimides and furans.6–9 At high temperatures, the rate of the retro-Diels–Alder reaction exceeds the rate of the forward reaction, and eventually net bond dissociation is observed as the equilibrium shifts to the endothermic side of the reaction. This results in the loss of cross-linkage in the polymer network enabling the mobility of the molecular chains, leading to a decrease in viscosity for reprocessing. Upon cooling, the equilibrium shifts to the right (Fig. 1a), leading to the reformation of cross-links and repossession thermoset-like properties.9
In contrast, associative CANs do not depolymerize on heating, and the bond dissociation only occurs when new bonds are formed. As a result, such materials have fixed cross-linking density and are covalently dynamic (Fig. 1). In 2005, Scott and coworkers reported the first associative CAN based on photo-mediated reversible cleavage involving the addition fragmentation chain transfer reaction of free radicals using allyl sulfide moieties.6,10
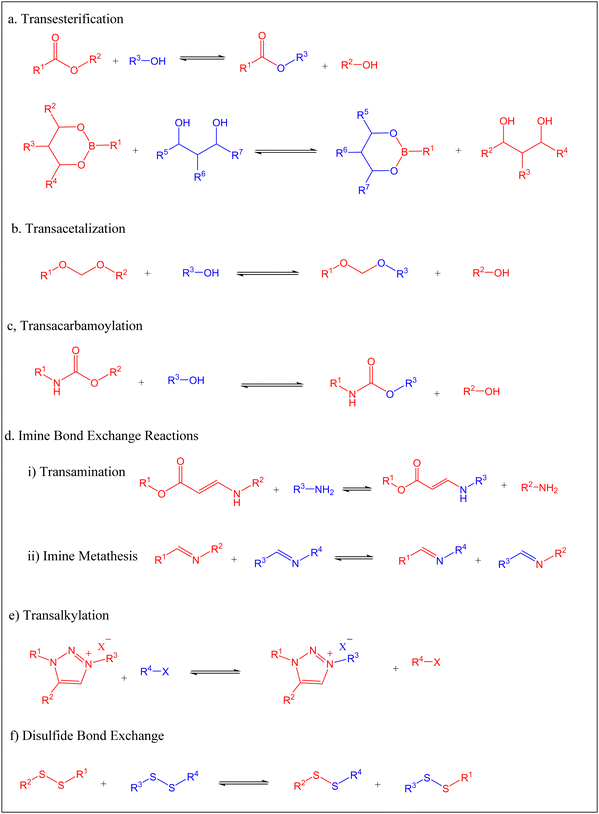
The past decades have witnessed intensive development of associative CAN materials, many of which rely on trans-esterification, trans-carbamoylation, and acetal and imine bond exchange reactions.11–14 In 2011, Leibler and coworkers studied the viscosity–temperature relationship of an associative CAN polymer network made via the catalytic esterification of diglycidyl ether of bisphenol A (DGEBA) and a mixture of fatty carboxylic acids, using an epoxy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COOH ratio of 1
COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The synthesized polyol/polyester network exhibited a unique Arrhenius-like viscosity–temperature relationship similar to vitreous silica.15 This ground-breaking finding in the field of polymer science gave rise to a new class of polymers with associative CAN cross-links which was named ‘vitrimers’.11,16 This marked one of the significant achievements in polymer science for the generation of recyclable/repairable thermosets.17 However, continued reliance on petroleum-based feedstocks has significantly contributed to environmental problems such as emission of greenhouse gases (GHGs) and environmental degradation.
1. The synthesized polyol/polyester network exhibited a unique Arrhenius-like viscosity–temperature relationship similar to vitreous silica.15 This ground-breaking finding in the field of polymer science gave rise to a new class of polymers with associative CAN cross-links which was named ‘vitrimers’.11,16 This marked one of the significant achievements in polymer science for the generation of recyclable/repairable thermosets.17 However, continued reliance on petroleum-based feedstocks has significantly contributed to environmental problems such as emission of greenhouse gases (GHGs) and environmental degradation.
To mitigate the environmental drawbacks that result from the disposal of fossil-based thermosets due to their non-degradability, non-recyclability, and non-renewability of fossil feedstock, researchers have taken advantage of vitrimer chemistry to develop biobased vitrimers to replace existing plastics or serve as novel materials. Bio-based vitrimers are prepared using biomass or biomass-derived materials as the building blocks.18–20 Numerous reviews have been conducted on bio-based vitrimers12,21,22 but, to the best of our knowledge, there is no exclusive review on lignin-based vitrimers, despite its abundance and rigidity that makes it a potential feedstock for thermoset-like materials. This review, therefore, focuses on the recent and noteworthy research activities on the utilization of lignin in bio-based vitrimers and vitrimer-like materials. Some chemical modifications of lignin are highlighted along with the thermo-mechanical properties of the reported lignin-based vitrimers.
2. Lignin and its modifications
Lignin is the second most abundant naturally occurring terrestrial polymer that typically constitutes about 15–30% by weight of dry lignocellulosic biomass.23,24 Structurally, it is a cross-linked complex heteropolymer most often consisting of guaiacyl (G), syringyl (S), or p-hydroxyphenyl (H) building units24,25 that result from the oxidative polymerization of three monolignols, i.e. coniferyl alcohol, sinapyl alcohol and p-coumaryl alcohol, respectively (Fig. 2).26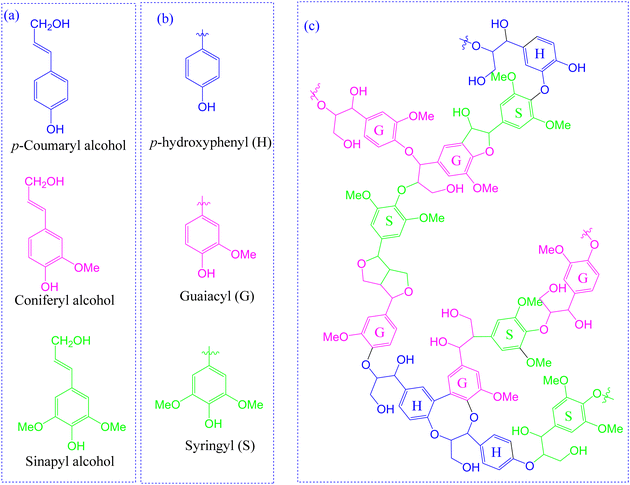 | ||
| Fig. 2 Chemical structure of lignin and its building units.23,25 | ||
As is well acknowledged in the literature, structural lignins can be categorized as hardwood, softwood, or non-wood (grass) lignin.27 Hardwood lignin is mainly composed of G and S units while softwood lignin consists of almost exclusively G units with small quantities of H units. In contrast, non-wood lignin is most often derived from all the three units.28–30 Moreover, varying random inter-linkages of these monolignols by C–C and C–O–C bonds results in structural differences exhibited by various lignins, for instance hardwood lignin displays a linear structure while a network structure is displayed by softwood lignin. Whereas a higher content of β-O-4 linkage is present in grass and hardwood lignin, higher C–C linkages (5–5, β–β, β-5, β-1) are observed in softwood lignin as shown in Table 1.
| Monolignol (%) | Proportions of linkages (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C–O–C | C–C | |||||||||
| H | G | S | β-O-4 | α-O-4 | 4-O-5 | 5–5 | β–β | β-5 | β-1 | |
| Softwood | <5 | >95 | 0 | 43–50 | 5–7 | 4 | 5–7 | 2–6 | 9–12 | 1–9 |
| Hardwood | 0–8 | 25–50 | 46–75 | 50–65 | <1 | 6–7 | <1 | 3–12 | 3–11 | 1–7 |
| Grass | 5–33 | 33–80 | 20–54 | 74–84 | n.d. | n.d. | n.d. | 1–7 | 5–11 | n.d. |
The first step in the valorization of lignin is its isolation from lignocellulosic biomass where several methods such as hydrolysis, Kraft, soda, organosolv, and enzymatic hydrolysis processes have been used.31,33–35 Since different parameters such as temperature, reagents, pH, solvents, pressure, and time are applied in these methods, the chemical properties and structure of a lignin are greatly dependent on the isolation technique. Consequently, these conditions result in varying structures and molecular weights of lignins.33,36 About 85% of total lignin production in the world is via a Kraft process.34 In this method, white liquor (a mixture of sodium sulfide and sodium hydroxide) is used at 150–180 °C for about 2 h.33,37 The isolated lignin usually contains traces of sodium and sulfur, with molecular weights depending on the source of biomass. In the sulfite pulping process, sulfites of calcium or magnesium are employed at varying pH levels and the isolated lignins (lignosulfonates) are charged, sulfonated, and have higher molecular weights than kraft lignins.38 Organosolv is another key isolation method where organic solvents such as ethanol, methanol, and acetone, with or without acid catalyst, are used at varying temperatures.39 Compared to kraft and sulfonate lignins, organosolv lignin is more chemically pure with a relatively low molecular weight.40,41 Other methods like enzymatic hydrolysis of lignocellulose followed by dioxane/water (94/4, v/v) extraction, co-solvent enhanced lignocellulosic fractionation (THF/acidic water), ammonia fiber explosion, etc. are also used.33,34,42,43 The average molecular weights of lignin vary depending on the source and the method of isolation as outlined in Table 2.
| Species | Isolation method | M w g mol−1 | M n g mol−1 | D | OH content in mmol g−1 | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Phenolic | Aliphatic | COOH | ||||||
| EMAL: enzymatic mild acidolysis lignin; CEL: cellulolytic enzyme lignin; MWL: milled wood lignin; CELF: cosolvent enhanced lignocellulosic fractionation; n.r.: not reported. | ||||||||
| Miscanthus | Organosolv | 7060 | 4690 | 1.51 | 3.08 | 1.19 | 0.22 | 44 |
| MWL | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
8300 | 1.65 | 1.66 | 4.00 | 0.13 | ||
| Eucalyptus | EMAL | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8700 | 3.7 | ∼1.0 | n.r. | 0.03 | 45 |
| MWL | 6700 | 2600 | 2.6 | ∼0.9 | n.r. | 0.04 | ||
| CEL | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
5500 | 3.1 | ∼0.7 | n.r. | 0.04 | ||
| Southern pine (normal wood) | EMAL | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
9700 | 5.9 | ∼1.4 | n.r. | 0.12 | 45 |
| MWL | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4700 | 3.2 | ∼1.6 | n.r. | 0.15 | ||
| CEL | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7500 | 3.9 | ∼1.3 | n.r. | 0.12 | ||
| White fir | EMAL | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6300 | 8.2 | ∼1.6 | n.r. | 0.20 | 45 |
| MWL | 8300 | 2800 | 3.0 | ∼1.9 | n.r. | 0.20 | ||
| CEL | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
4700 | 4.6 | ∼1.2 | n.r. | 0.20 | ||
| Poplar | CELF lignin | 1289 | 429 | 3.0 | 1.9 | 0.55 | 1.1 | 43 |
| Organosolv | 1718 | 652 | 2.7 | 2.65 | 1.25 | 0.70 | ||
| Kraft lignin | 2533 | 573 | 4.4 | 2.8 | 1.60 | 0.85 | ||
Owing to its rich carbon content, mechanical strength, thermal and chemical stability, lignin is a suitable bio-based substitute for petrochemical-derived feedstock in polymer synthesis.46,47 Furthermore, the multifunctionality of lignin makes it compatible with a wide range of other compounds,48,49 as well as conversion to different functional materials through depolymerization or chemical modifications.49–52
Although lignin can be utilized without modification in low-value applications, increased focus on renewable bio-based materials has led to the development of various chemical modification techniques aimed at improving its properties and miscibility with other polymeric materials.53,54 Lignin is modified with different functional groups to fulfill the requirement of vitrimer synthesis. One of the most employed modifications is the introduction of a carboxylic acid group by taking advantage of catalytic transesterification reactions between the OH groups in lignin and anhydrides.55–57 Carboxylation can also be achieved by reacting lignin with ozone, a reaction that is accompanied by a decrease in the molecular weight of lignin due to the cleavage of its aromatic rings.58,59 As shown in Table 3, ozonolysis of pyrolytic lignin (PL) and organosolv lignin (OL) under ambient temperature resulted in a 29% and 71% decrease in the average molecular weight of PL and OL respectively, alongside the generation of a mixture of esters, (di)carboxylic acids, and acetals and esters, via esterification, oxidative ring opening and acetal synthesis pathways.60 Using sodium hypochlorite (NaOCl), Wang et al. demonstrated the oxidation of steam explosion lignin to polycarboxylated lignin. Their results exhibited a decrease in the molecular weight accompanied by disruption of the aromatic rings of lignin. The decrease in molecular weight was attributed to the structural disruption and fragmentation of the lignin structure.61
| Lignin type | Before ozonolysis (g mol−1) | After ozonolysis (g mol−1) | % decrease in Mw | Ref. | ||
|---|---|---|---|---|---|---|
| M w | M n | M w | M n | |||
| a For ozonolysis performed at pH 3. b At pH 7. c At pH 12. | ||||||
| Pine-derived pyrolytic lignin | 650 | 420 | 395 | 325 | 39 | 62 |
| Pyrolytic lignin | 786 | — | 555 | — | 29 | 60 |
| Ethanosolv (from walnut shells) | 2947 | — | 854 | — | 71 | |
| Butanosolv (from walnut shells) | 4464 | — | 1426 | — | 68 | |
| Alkali lignin (from hardwood pulping liquor) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 116 |
7146 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667a 667a |
5154a | 24 | 63 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474b 474b |
5384b | 12 | ||||
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192c 192c |
5946c | 6.5 | ||||
Other reported modifications of lignin involve the introduction of an epoxy functional group via a reaction between lignin and epichlorohydrin in the presence of a base catalyst;64,65 and the introduction of an amino group which can be achieved by reacting an epoxidized lignin with an amine,66 oxazolidinone followed by decarboxylation,67 (3-aminopropyl)triethoxysilane,68 or with formaldehyde and an amine (Mannich reaction) in which case the amino group is introduced at the C5 position of lignin sub-units (Fig. 3).69–71
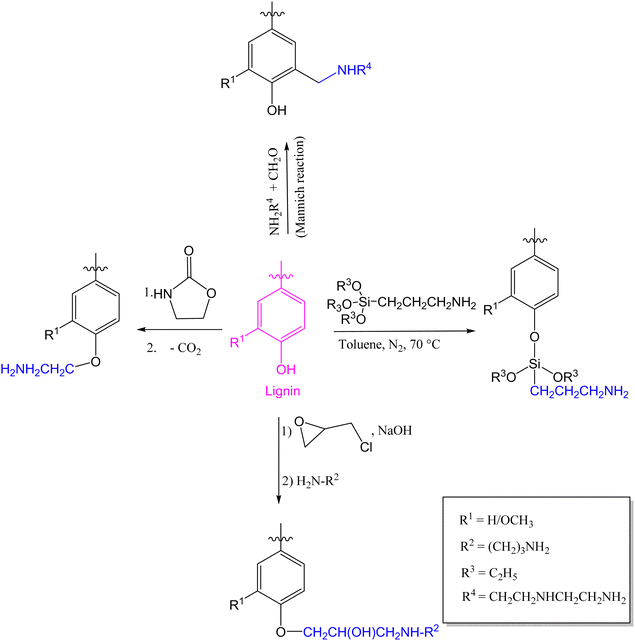 | ||
| Fig. 3 Various lignin amination techniques.66–68,70 | ||
Due to its high energy content (∼26.6 kJ g−1),72 rigidity, and renewability, the potential for industrial application of lignin is enormous. Its rich aromatic content makes it a viable substitute for fossil-based chemicals. During biomass pretreatment, the waste stream is mainly composed of lignin that frequently ends up in low-value applications such as additives, adhesives, dispersants, and direct combustion fuel.73 With increased demand for cellulosic biofuel globally, alongside the growth in demand for lignin-based products, it is estimated that the annual production of lignin will increase to 225 million tons by 2030,74 and thus it is important to develop valorization technologies that are cost effective and sustainable for biorefineries. For instance, integrating lignin valorization into biofuel production from lignocellulosic biomass can reduce waste management cost and generate new revenue, thereby rendering biomass-derived fuels and other platform chemicals economically competitive.
3. Lignin-based vitrimers
In this section, reported lignin-based vitrimers will be categorized and discussed based on their dynamic bond exchange reactions (Fig. 4). The notable thermo-mechanical properties will be highlighted alongside their potential application.3.1 Transesterification vitrimers
Among the various chemistries enabling bond exchange in covalent adaptable networks (CANs), thermally activated transesterification reaction (TER) has attracted considerable interest. This is partly due to the ease of implementation75 and the abundance of commercially available feedstock.58In 2018, Zhang and coworkers reported a fully bio-based vitrimer from ozonated kraft lignin (Oz-L) and di-epoxidized sebacic acid (Se-EP) using zinc acetoacetonate (Zn(acac)2) as the catalyst (Scheme 1). Ozone-oxidation of lignin resulted in the degradation of some aromatic rings as well as the introduction of more carboxylic acid moieties.58 The Oz-L was cured with Se-EP at 150 °C and 190 °C for 1 h and 2 h respectively, to form vitrimers whose glass transition temperature (Tg) (95–133 °C), storage modulus (>1 GPa) and tensile strength (5.1–12.9 MPa) increased with increasing lignin content. This trend was related to the rigidity of polymer backbones and crosslinking density which have a direct relationship with lignin content. The synthesized networks exhibited a clear stress relaxation at 160 °C and 200 °C, but there was no clear relaxation at a low temperature (120 °C). At 200 °C, relaxation times decreased from 1290 s to 81 s when epoxy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (aromatic OH + carboxylic OH) ratio (R) decreased from 1
(aromatic OH + carboxylic OH) ratio (R) decreased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 to 1
0.75 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. This was attributed to the presence of more unreacted carboxylic acid groups in higher Oz-L content (higher R) that efficiently induced transesterification reactions. The self-healing behavior of surface scratched samples and physical recycling by hot press at 190 °C were demonstrated in this work. The synthesized samples also exhibited good shape memory behavior and potential use as recoverable adhesives. When the Z-shaped and W-shaped samples were flattened at 80 °C, followed by quick cooling, the samples regained their original shapes after reheating to 80 °C. However, the rate of recovery decreased with increased lignin content i.e., about 97%, 94%, and 87% for vitrimer samples with R values of 1
1.5. This was attributed to the presence of more unreacted carboxylic acid groups in higher Oz-L content (higher R) that efficiently induced transesterification reactions. The self-healing behavior of surface scratched samples and physical recycling by hot press at 190 °C were demonstrated in this work. The synthesized samples also exhibited good shape memory behavior and potential use as recoverable adhesives. When the Z-shaped and W-shaped samples were flattened at 80 °C, followed by quick cooling, the samples regained their original shapes after reheating to 80 °C. However, the rate of recovery decreased with increased lignin content i.e., about 97%, 94%, and 87% for vitrimer samples with R values of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 respectively. This trend was attributed to the fact that as lignin content increased, there was restrained mobility of the vitrimer networks which resulted from the increased modulus at 80 °C. The level of Se-EP/Oz-L adhesion on aluminum sheets was comparable to some other commercial epoxy adhesives, with the distinct ability to thermally rebond at 190 °C after a cohesive failure. When Se-EP and Oz-L (ratio 1
0.75 respectively. This trend was attributed to the fact that as lignin content increased, there was restrained mobility of the vitrimer networks which resulted from the increased modulus at 80 °C. The level of Se-EP/Oz-L adhesion on aluminum sheets was comparable to some other commercial epoxy adhesives, with the distinct ability to thermally rebond at 190 °C after a cohesive failure. When Se-EP and Oz-L (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were loaded and cured onto the surfaces of coarsened sheets of aluminium, 6.5 MPa of lap shear strength that was comparable to other epoxy-based adhesives was obtained. For instance, 4–6 MPa of lap shear strength was exhibited by DGEBA epoxy-based adhesive with aluminium A1050.76
1) were loaded and cured onto the surfaces of coarsened sheets of aluminium, 6.5 MPa of lap shear strength that was comparable to other epoxy-based adhesives was obtained. For instance, 4–6 MPa of lap shear strength was exhibited by DGEBA epoxy-based adhesive with aluminium A1050.76
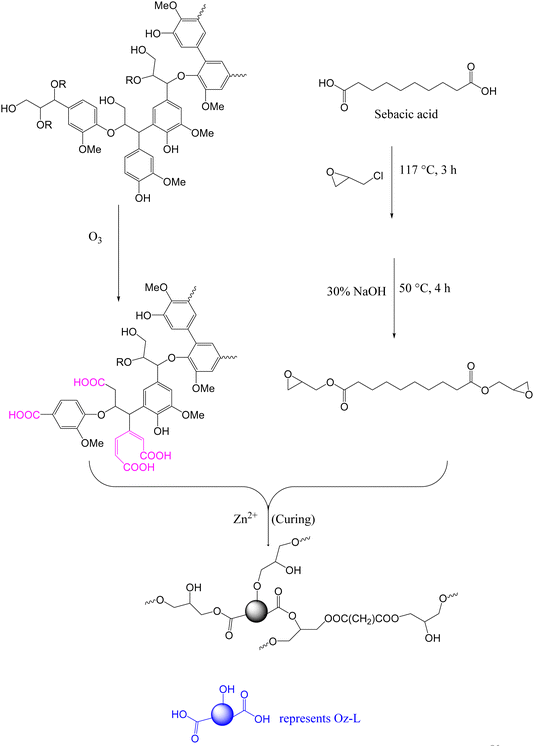 | ||
| Scheme 1 Schematic representation of Se-EP/Oz-L vitrimer synthesis.58 | ||
Zheng and coworkers took advantage of the photothermal conversion capability of lignin to prepare a photo-remoldable vitrimer from the reaction between epoxy modified kraft lignin (EML) with poly(ethylene glycol) bis(carboxymethyl) ether (PEG-Bis(CMC)) in presence of zinc acetate catalyst.77 In this work, coniferous wood-derived kraft lignin was first modified by epoxidation using epichlorohydrin. The introduction of the epoxy group was demonstrated by the FTIR absorption peaks at around 910 cm−1 associated with C–O–C (ether) and 1030 cm−1 for the C–O bond attached alkyl group, which was further confirmed by thermogravimetric (TG) and 1H NMR analyses. Using an acid–acetone titration method,77 the epoxy value of the resultant EML was determined as 5.5 mmol g−1. At the epoxy/COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EML/PEG-Bis(CMC) mixtures were cured at 120 °C for 4 h, and the obtained vitrimers were found to be hydrophilic with contact angles of 45.5° and 28.5° for vitrimer with low (P600) and high (P2000) molecular weight of PEG-Bis(CMC) respectively. The hydrophilicity was due to the presence of PEG-Bis(CMC), whose content inversely affects the crosslinking density and consequently the contact angle with water. The synthesized samples demonstrated photothermal conversion ability. When subjected to infrared laser irradiation with 1.00 W cm−2 power density, the surface temperature of the vitrimers increased rapidly to 170–231 °C, while pure PEG-Bis(CMC) was inert to infrared light. The conjugation and aromatic rings in the EML were the two factors attributed to this excellent photothermal property of the synthesized lignin-based vitrimers. Reprocessing tests showed that in situ light-controlled method was more convenient and energy efficient compared to hot pressing. During hot pressing, 22 kW h of electrical energy were consumed for 2 h at 150 °C to remold the sample, whereas only 20 min and 0.02 kW h of electrical energy were used in light-controlled remolding procedure under 808 nm IR laser with a power density of 1 W cm−2. Thermal–mechanical properties of the vitrimer networks were performed using TGA and tensile testing machine. The tensile strength of the samples increased with an increase in the molecular weight of the PEG-Bis(CMC), i.e., 1.45 MPa and 4.52 MPa for vitrimer with P600 and P2000 PEG-Bis(CMC), respectively. The tensile strength of the reprocessed samples was found to be 84.1% and 55% of the virgin samples for hot press and in situ light-control method, respectively. All synthesized networks exhibited good thermal stability with the onset and maximum degradation temperatures of 280 °C and 374 °C, respectively for the vitrimer with P600 PEG-Bis(CMC), and 315 °C and 392 °C respectively for vitrimer with P2000 PEG-Bis(CMC). The potential use of the synthesized vitrimers as an adhesive was demonstrated using two sheets of glass that could withstand a pulling force greater than 9.0 N. The adhesive action of this lignin-based epoxy vitrimer was attributed to the presence of the methoxy groups, phenolic OH groups and free C5 site on the aromatic ring, with a possibility of further cross-linking.
1, EML/PEG-Bis(CMC) mixtures were cured at 120 °C for 4 h, and the obtained vitrimers were found to be hydrophilic with contact angles of 45.5° and 28.5° for vitrimer with low (P600) and high (P2000) molecular weight of PEG-Bis(CMC) respectively. The hydrophilicity was due to the presence of PEG-Bis(CMC), whose content inversely affects the crosslinking density and consequently the contact angle with water. The synthesized samples demonstrated photothermal conversion ability. When subjected to infrared laser irradiation with 1.00 W cm−2 power density, the surface temperature of the vitrimers increased rapidly to 170–231 °C, while pure PEG-Bis(CMC) was inert to infrared light. The conjugation and aromatic rings in the EML were the two factors attributed to this excellent photothermal property of the synthesized lignin-based vitrimers. Reprocessing tests showed that in situ light-controlled method was more convenient and energy efficient compared to hot pressing. During hot pressing, 22 kW h of electrical energy were consumed for 2 h at 150 °C to remold the sample, whereas only 20 min and 0.02 kW h of electrical energy were used in light-controlled remolding procedure under 808 nm IR laser with a power density of 1 W cm−2. Thermal–mechanical properties of the vitrimer networks were performed using TGA and tensile testing machine. The tensile strength of the samples increased with an increase in the molecular weight of the PEG-Bis(CMC), i.e., 1.45 MPa and 4.52 MPa for vitrimer with P600 and P2000 PEG-Bis(CMC), respectively. The tensile strength of the reprocessed samples was found to be 84.1% and 55% of the virgin samples for hot press and in situ light-control method, respectively. All synthesized networks exhibited good thermal stability with the onset and maximum degradation temperatures of 280 °C and 374 °C, respectively for the vitrimer with P600 PEG-Bis(CMC), and 315 °C and 392 °C respectively for vitrimer with P2000 PEG-Bis(CMC). The potential use of the synthesized vitrimers as an adhesive was demonstrated using two sheets of glass that could withstand a pulling force greater than 9.0 N. The adhesive action of this lignin-based epoxy vitrimer was attributed to the presence of the methoxy groups, phenolic OH groups and free C5 site on the aromatic ring, with a possibility of further cross-linking.
Apart from hot pressing, lignin-based transesterification vitrimers can also be recycled by using chemical means. Du and coworkers used epoxidized and carboxylated enzymatic lignin to synthesize thermally stable vitrimers (onset degradation temperature, Td5% of 262.54–285.43 °C) with Tg of 100–102.5 °C and underwent dynamic reversible transformation in ethylene glycol at high temperature (190 °C), with an activation energy of 18.12 kJ mol−1. Epoxidized enzymatic lignin (EP-EL) and carboxylated lignin (CA-EL) were obtained by modifying lignin with epichlorohydrin and maleic anhydride, respectively. EP-EL and CA-EL were mixed in different ratios, with the addition of an appropriate amount of polyethyelene glycol (PEG 400).78 The mechanical strength (48–68 MPa) and elongation at break (60–80%) of the prepared catalyst-free vitrimers improved with increase in EP-EL content, indicating the possibility of complete polymerization and increased cross-linking of the material at elevated temperature. The addition of a catalyst led to reduced binding strength within the network, resulting in decreased thermal stability in comparison with the catalyst-free networks. However, the observed decrease in the energy storage modulus upon the addition of a catalyst was not accounted for. Recycling through hot pressing was done under 4 MPa at 150 °C for 15 min. At the same time, solvent resistance was demonstrated using 1 M hydrochloric acid (HCl), distilled water (H2O), dichloromethane (DCM), ethylene glycol (EG), tetrahydrofuran (THF), toluene (TOL) and dimethylformamide (DMF). When catalyst-free samples with equal amounts of EP-EL, CA-EL and PEG400 (W4440) were immersed in each of the solvent (i.e., HCl, H2O, DCM, EG, THF, TOL, and DMF) for 24 h, they exhibited stability in most of the solvents, which rendered them suitable for adhesion, coating, and rubber bonding. When vitrimers were applied to glass, the coating easily formed into different shapes without the use of a mold and was only dislodged using 1 M NaOH at 90 °C, and recovered by neutralizing the solution with HCl, indicating the instability (thus chemical recyclability) of ester linkages in alkaline medium. Due to the photothermal properties of lignin, the vitrimers demonstrated the ability to bond to other materials and adhesively to themselves.78
Small units derived from oxidative polymerization of lignin are used in the preparation of transesterification-based vitrimers. For example, More and coworkers synthesized lignin-derived vanillic acid (OxL-COOH) that was cured with poly(ethylene glycol) diglycidyl ether (PEG-epoxy) to form thermally stable polymer films (5% weight loss temperature at about 270 °C and a maximum weight loss rate at about 390 °C), with noteworthy swelling in an alkaline medium.79 Vanillic acid was obtained by oxygen-oxidation of softwood kraft lignin under alkaline conditions, followed by ozonolysis. The optimized conditions of 130 °C and 40 min were used to sequentially oxidize kraft lignin, followed by ozone-oxidation for 2 h at 80 °C. The application of ozone, at a rate of 5000 mg h−1, increased the carboxylic content from 1.41 mmol g−1 to 2.06 mmol g−1 after 2 h of ozone oxidation. Using various epoxy/COOH ratios (i.e., 1.5, 1.3 and 1.0), three sets of vitrimers were synthesized by curing a mixture of OxL-COOH and PEG-epoxy at the optimized temperature of 110 °C for 4 h. The storage modulus (7.06 × 107–2.08 × 108 Pa) and Tg of the obtained films increased with the decrease in epoxy/COOH ratio. As the OxL-COOH increased, lignin content in the synthesized samples also increased, whose stiffness and myriad of reactive groups contributed to the observed trend. The evidence for dynamic ester linkages was demonstrated by stress relaxation of the synthesized samples at room temperature. The sample with epoxy/COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the fastest rate of relaxation, while that with 1.5
1 exhibited the fastest rate of relaxation, while that with 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had the slowest relaxation rate which was reportedly due to the presence of unreacted hydroxyl groups that resulted from epoxy ring opening. The self-healing behavior of razor-cracked films was studied by heating the samples at 200 °C for 30 min, and changes in the crack were monitored by an optical microscope. The sample with an epoxy/COOH ratio of 1
1 had the slowest relaxation rate which was reportedly due to the presence of unreacted hydroxyl groups that resulted from epoxy ring opening. The self-healing behavior of razor-cracked films was studied by heating the samples at 200 °C for 30 min, and changes in the crack were monitored by an optical microscope. The sample with an epoxy/COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 demonstrated a rapid repair process with a 54.1% decrease in crack width after 30 min (from 17.5 μm to 8.03 μm). The crack width of samples with epoxy/COOH ratios of 1.3
1 demonstrated a rapid repair process with a 54.1% decrease in crack width after 30 min (from 17.5 μm to 8.03 μm). The crack width of samples with epoxy/COOH ratios of 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.5
1 and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 decreased from 10.33 μm to 5.01 μm and 8.37 μm to 6.09 μm respectively. From these results, the epoxy/COOH ratio of 1
1 decreased from 10.33 μm to 5.01 μm and 8.37 μm to 6.09 μm respectively. From these results, the epoxy/COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 represented the most efficient trans-esterification vitrimers, which is in line with other published studies.80
1 represented the most efficient trans-esterification vitrimers, which is in line with other published studies.80
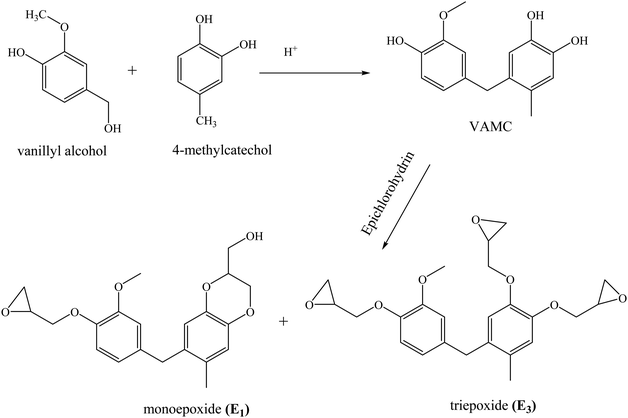
Abu-Omar and Zhao synthesized an epoxy prepolymer from lignin-derived 4-methylcatechol and vanillyl alcohol.81 The product derived from the condensation reaction between vanillyl alcohol and 4-methyl catechol yielded a mixture of triepoxide (E3) and monoepoxide (E1) upon reaction with epichlorohydrin (Scheme 2). The ratio of E1 and E3 was tunable by changing the reaction conditions. The cross-linking density and Tg of vitrimers (11–23 °C) obtained by curing this epoxide mixture with a mixture of fatty acids trimers and dimers increased with decrease in the E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E3 ratio. For example, a cross-linked structure was not formed from an epoxide mixture with 14% triepoxide (i.e., E1
E3 ratio. For example, a cross-linked structure was not formed from an epoxide mixture with 14% triepoxide (i.e., E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E3 of 86
E3 of 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14), while networks that exhibited stress relaxation were obtained when E3 in the epoxide mixture increased to 40% (60
14), while networks that exhibited stress relaxation were obtained when E3 in the epoxide mixture increased to 40% (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and 64% (36
40) and 64% (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64) after curing at 65 °C, 120 °C and 160 °C for 4 h, 18 h and 4 h respectively. Vitrimers with E1
64) after curing at 65 °C, 120 °C and 160 °C for 4 h, 18 h and 4 h respectively. Vitrimers with E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E3 ratio of 60
E3 ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 exhibited a relaxation time of 456 s at 120° and an activation energy (Ea) of 24.3 kJ mol−1, while that with E1
40 exhibited a relaxation time of 456 s at 120° and an activation energy (Ea) of 24.3 kJ mol−1, while that with E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E3 of 36
E3 of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 had a relaxation time of 10
40 had a relaxation time of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 s at 130 °C, and 59.5 kJ mol−1 of the activation energy. The lower Ea associated with samples bearing E1
693 s at 130 °C, and 59.5 kJ mol−1 of the activation energy. The lower Ea associated with samples bearing E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E3 ratio of 60
E3 ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 was due to lower crosslinking density alongside the higher number of free OH groups capable of accelerating TER. As E3 content increased, a higher crosslinking density occurred and the storage modulus (>103 MPa), tensile strength (2.7–25.1 MPa), and thermal stability (as shown by onset degradation temperature, 300–354 °C) of the synthesized vitrimer samples also increased. The self-healing of cut pieces and reprocessability through melt-molding at 100 °C were also demonstrated in this study.81
40 was due to lower crosslinking density alongside the higher number of free OH groups capable of accelerating TER. As E3 content increased, a higher crosslinking density occurred and the storage modulus (>103 MPa), tensile strength (2.7–25.1 MPa), and thermal stability (as shown by onset degradation temperature, 300–354 °C) of the synthesized vitrimer samples also increased. The self-healing of cut pieces and reprocessability through melt-molding at 100 °C were also demonstrated in this study.81
Xue et al. reported lignin-based epoxy vitrimers with high mechanical strength, which were prepared by curing different ratios of DGEBA and GEL (glycidyl etherified enzymatic hydrolysis lignin) with dodecane dicarboxylic acid in the presence of zinc catalyst.82 GEL, which was made by reacting enzymatic hydrolysis lignin (EL) with epichlorohydrin in the presence of tert-butylammonium bromide catalyst, which was dispersed in DMF alongside DGEBA to produce various mixtures with GEL/DGEBA ratios of 0/1, 1/8, 1/4, 1/2, 1/1, 2/1, and 1/0. These mixtures were cured with dodecanedioic acid (DEA) for 4 h and 6 h at 120 °C and 160 °C, respectively, using epoxy/COOH molar ratio of 1/1 for all the samples. The tensile strength (4.9–39.5 MPa), cross-linking density (60–94.7%), Young's modulus (32.4–931.1 MPa), and the storage modulus (502.6–1004.6 MPa) of the synthesized vitrimers increased as the GEL content increased from 0% to 72.1% in GEL/DGEBA ratio 0/1 and 1/0, respectively. In relation to pure DGEBA vitrimer, the Tg of vitrimers decreased slightly with small replacement of GEL, i.e. from 25.5 °C to 23.1 °C in samples with 0% and 11.1% GEL content. This phenomenon was due to the presence of methoxy groups and flexible linkages (such as β-0-4 and β-5) in the structure of lignin that tend to lower the Tg of the cured samples. The presence of methoxy groups enhances the degree of rotation of the polymer network, thus lowering the Tg.83,84 However, the continuous increase in lignin content was accompanied by an increase in the Tg due to an increase in the crosslinking density. The effects of different carboxylic acids on the mechanical properties of the vitrimers were studied using the sample GEL/DGEBA – 1/2 as the control, and introducing 1,2,3-pentanetricarboxylic acid (PTA) to the curing system. The results indicated that the tensile strength increased gradually, with the highest tensile strength (46.8 MPa) attained with the DEA/PTA molar ratio of 1/1. This was due to an increase in the cross-linking density in the vitrimer network. The synthesized vitrimers demonstrated excellent self-healing behavior with cracked samples recording an 80% repair in 5 min at 160 °C. Furthermore, the healing efficiency of the samples fully cut perpendicular to the tensile direction decreased as the lignin content in the sample increased. As the lignin content increased, the movement of the polymer chains was hampered, thus hindering the recovery of the dynamic covalent network. The vitrimers exhibited good reprocessability by retaining more than 80% tensile strength after two cycles of hot pressing at 190 °C, shape memory at 100 °C, and good thermal stability (temperature at 10% weight loss of 290.2–328.3 °C) that increased with increasing lignin content.82
In a different study that aimed at investigating how the structural characteristics of lignin affect the performance of vitrimers, Xue's research group prepared lignin-based epoxy vitrimers (LEVs) from fractionated lignin and sebacic acid and compared their mechanical performance with those of unfractionated lignin.85 Enzymatically hydrolyzed lignin was first fractionated using ethylacetate, ethanol, and acetone. The polydispersity (PDI) of all the fractionated lignins were lower than that of unfractionated lignin (Table 4), with an ethanol-soluble fraction having the highest yield (36.73%) and phenolic OH content (3.34 mmol g−1).85 Epoxidation was then accomplished using epichlorohydrin, before curing with sebacic acid using an epoxy to COOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Whereas the vitrimer from unfractionated lignin (LEVR) was so brittle that mechanical testing could not be performed, the tensile strength of ethylacetate-soluble and ethanol-soluble lignin-based vitrimers (LEVEA and LEVE) were similar (i.e. 38.25 MPa and 39.82 MPa respectively). In comparison to LEVEA and LEVE, the acetone-soluble fractionated-lignin-based vitrimer (LEVA) had a lower tensile strength (20.79 MPa). The higher tensile strength in LEVEA and LEVE was due to lower lignin molecular weight and higher epoxy content that was crucial in the reaction with acidic groups thus leading to a higher cross-linking density. As shown in Table 4, the toughness, Young's modulus, and storage modulus decreased with decrease in molecular weight of fractionated lignin. In contrast, the DSC-based Tg increased with an increase in molecular weight (from 40.4 °C in LEVE to 66.8 °C in LEVA), which was attributed to structural differences in lignin. Higher hydroxyl groups content in LEVEA resulted in faster stress relaxation than LEVA at 180 °C. Reprocessability efficiency, measured by comparing the tensile strength of hot-pressed samples at 160 °C and 5 MPa, was significantly lower in LEVA (31.2%) than in LEVE (56.71%) and LEVEA (55.26%) due to low reactivity associated with higher molecular weight lignin that results from rigidity of the structure and higher steric hindrance. Furthermore, all samples exhibited good thermal stability (with Tmax 356.92–394.15 °C) and excellent self-repair at 160 °C with over 90% recovery in only 1 min.
1. Whereas the vitrimer from unfractionated lignin (LEVR) was so brittle that mechanical testing could not be performed, the tensile strength of ethylacetate-soluble and ethanol-soluble lignin-based vitrimers (LEVEA and LEVE) were similar (i.e. 38.25 MPa and 39.82 MPa respectively). In comparison to LEVEA and LEVE, the acetone-soluble fractionated-lignin-based vitrimer (LEVA) had a lower tensile strength (20.79 MPa). The higher tensile strength in LEVEA and LEVE was due to lower lignin molecular weight and higher epoxy content that was crucial in the reaction with acidic groups thus leading to a higher cross-linking density. As shown in Table 4, the toughness, Young's modulus, and storage modulus decreased with decrease in molecular weight of fractionated lignin. In contrast, the DSC-based Tg increased with an increase in molecular weight (from 40.4 °C in LEVE to 66.8 °C in LEVA), which was attributed to structural differences in lignin. Higher hydroxyl groups content in LEVEA resulted in faster stress relaxation than LEVA at 180 °C. Reprocessability efficiency, measured by comparing the tensile strength of hot-pressed samples at 160 °C and 5 MPa, was significantly lower in LEVA (31.2%) than in LEVE (56.71%) and LEVEA (55.26%) due to low reactivity associated with higher molecular weight lignin that results from rigidity of the structure and higher steric hindrance. Furthermore, all samples exhibited good thermal stability (with Tmax 356.92–394.15 °C) and excellent self-repair at 160 °C with over 90% recovery in only 1 min.
| Vitrimer sample | LEVEA | LEVE | LEVA | LEVR |
|---|---|---|---|---|
| M w of lignin (g mol−1) | 1471 | 2729 | 4957 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016 016 |
| PDI | 1.60 | 1.95 | 2.48 | 2.63 |
| Tensile strength (MPa) | 38.56 | 39.82 | 20.79 | — |
| Young's modulus (GPa) | 1.89 | 1.46 | 1.18 | — |
| Toughness (kJ m−3) | 89.2 | 126.36 | 22.56 | — |
| Storage modulus at 25 °C (GPa) | 1.22 | 1.03 | 0.92 | — |
| T g (°C) | 48.1 | 40.4 | 66.8 | — |
| Reprocessing efficiency (%) | 55.26 | 56.71 | 31.02 | — |
A fully bio-based vitrimer made from epoxidized enzymatic lignin and carboxylated enzymatic lignin, which was further used to synthesize recyclable epoxy asphalt, was reported by Song et al., in 2023.86 Enzymatic lignin (EL) was separately epoxidized and carboxylated by reacting with epichlorohydrin and maleic anhydride. The vitrimers obtained by mixing epoxidized lignin, carboxylated lignin, PEG 400 and zinc acetate at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 and cured at 150 °C and 170 °C for 4 h and 8 h, respectively, exhibited slow stress relaxation at 180 °C and Ea of 97.21 kJ mol−1. Though, as expected, the stress relaxation rate increased with increased temperature, and the minimum possible stress relaxation temperature was comparably higher than reported in other vitrimer systems. Using an impregnation method, the vitrimer exhibited higher gel content (>70%) and chemical resistance in 1 M HCl, dichloromethane, THF, DMF, and in ethyleneglycol. To prepare recyclable epoxy asphalt (ELEA), enzymatic lignin-based vitrimer (ELV) was mixed with base asphalt at 120 °C and cured at 150 °C and 170 °C for 4 h and 8 h, respectively. The prepared asphalt was found to meet the requirements set out for the road asphalt in the Technical Specification for the Construction of Asphalt Pavements (JTG F40-2004). As compared to the base asphalt (AH-70), ELEA exhibited significantly lower penetration (46.27–26.5 mm) and higher softening point (57.95–74.85 °C), which decreased and increased respectively, with increase in ELV. Good deformability at low temperatures was indicated by low Tg values. Furthermore, the tensile strength and viscosity increased as ELV content increased. However, the initial decomposition temperature increased when ELV content changed from 10% to 15% (i.e., 287 °C to 303 °C), then decreased as the content increased to 30% (i.e., from 303 °C at 15% to 267 °C at 30%). As shown in Table 5 below, the proposed lignin-modified asphalt has better performance in terms of penetration, temperature stability, and viscosity than some asphalt blended with petroleum-based polymers, owing to lignin thermal stability and rigidity.
0.06 and cured at 150 °C and 170 °C for 4 h and 8 h, respectively, exhibited slow stress relaxation at 180 °C and Ea of 97.21 kJ mol−1. Though, as expected, the stress relaxation rate increased with increased temperature, and the minimum possible stress relaxation temperature was comparably higher than reported in other vitrimer systems. Using an impregnation method, the vitrimer exhibited higher gel content (>70%) and chemical resistance in 1 M HCl, dichloromethane, THF, DMF, and in ethyleneglycol. To prepare recyclable epoxy asphalt (ELEA), enzymatic lignin-based vitrimer (ELV) was mixed with base asphalt at 120 °C and cured at 150 °C and 170 °C for 4 h and 8 h, respectively. The prepared asphalt was found to meet the requirements set out for the road asphalt in the Technical Specification for the Construction of Asphalt Pavements (JTG F40-2004). As compared to the base asphalt (AH-70), ELEA exhibited significantly lower penetration (46.27–26.5 mm) and higher softening point (57.95–74.85 °C), which decreased and increased respectively, with increase in ELV. Good deformability at low temperatures was indicated by low Tg values. Furthermore, the tensile strength and viscosity increased as ELV content increased. However, the initial decomposition temperature increased when ELV content changed from 10% to 15% (i.e., 287 °C to 303 °C), then decreased as the content increased to 30% (i.e., from 303 °C at 15% to 267 °C at 30%). As shown in Table 5 below, the proposed lignin-modified asphalt has better performance in terms of penetration, temperature stability, and viscosity than some asphalt blended with petroleum-based polymers, owing to lignin thermal stability and rigidity.
| Asphalt | Penetration (0.1 mm) | Softening (°C) | Ductility (cm) | Viscosity (Pa s) | Ref. |
|---|---|---|---|---|---|
| NPE – NH2-terminated polyethylene; LDPE – low density polyethylene; HDPE – high density polyethylene. | |||||
| Lignin-modified epoxy asphalt | 26.5–46.27 | 57.95–74.85 | — | 1.194–1.980 at 145 °C | 86 |
| NPE-modified asphalt | 59.1–64.3 | 49.8–51.8 | 197.5–313.5 | ≈−0.36 at 135 °C | 87 |
| Light-controlled asphalt | 52 | 62 | 25 | 2.2 at 135 °C | 88 |
| LDPE-modified asphalt | 62 | 60 | — | — | 89 |
| HDPE-modified asphalt | 52 | 84 | — | — | 89 |
Fractionation of kraft lignin (using solvent extraction) is often employed to improve its compatibility with other building blocks.90,91 Depending on the choice of solvent, lignin fractions with different chemical functionalities and molecular weights can be obtained. Using liquid–solid and microwave-assisted extraction of kraft lignin, Hakkarainen and his group synthesized flexible and recyclable polyester thermosets by mixing lignin fractions with citric acid and PEG. The Tg (4.4–8.7 °C), tensile strength (1.8–2.5 MPa), and Young's modulus (3.0–4.1 MPa) of the resultant polyester thermosets were lower than a polyester from the original lignin, i.e., 10.2 °C, 3.7 MPa and 9.9 MPa, respectively.92 This effect was due to higher interactions and more cross-linking associated with higher molecular weight lignin. As the molecular weight of lignin increased, the thermo-mechanical properties of the prepared samples were improved. However, the elongation at break of the synthesized samples from fractionated lignin (Mw 1800–3700 g mol−1) was higher (>204%) than from the original lignin (99.2%). Furthermore, better chemical recyclability was achieved within 20 min for samples from fractionated lignin in microwave-heated alkaline solution at 160 °C.
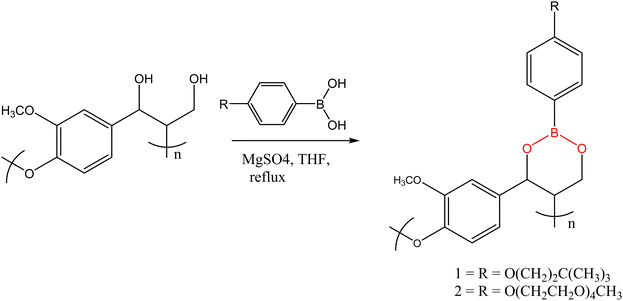
Though most reported transesterification vitrimers are based on carboxylic acid esters, synthesis of polymer networks with dynamic boronic acid-based esters has been reported in the literature.93–95 Lignin-based polymers containing boronate ester linkages was first reported by Lovine's research group in 2009 when they successfully modified a synthetic lignin polymer through introduction of arylboronate linkages,96 a method that was later applied to organsolv lignin. The synthetic lignin prepared contained 1,3-diols in the β-O-4 structural subunit, which were then reacted with arylbornonic acid to form cyclic boronate ester linkages. The Tg of boron-modified lignin polymers (101 °C and 43 °C for polymer 1 and 2, respectively Fig. 5) depended on the side-chain modification of lignin and arylboronic acid, with the plasticizing effect of polyether groups witnessed in significantly lower Tg in 2.
Using a “graft to” model, Lovine et al. developed graft copolymers by covalently linking organosolv lignin with borononic acid-end modified polycaprolactone (PCL) through reversible boronate ester linkages. Under mild dehydration, the organsolv lignin was reacted with arylboronic acid end-functionalized PCL to form lignin-PCL copolymers that exhibited three distinct thermal transitions at about 50 °C (endothermic melt), around 5 °C (exothermic PCL-based crystallization), and −10 °C to −60 °C related to PCL Tg temperature. At high lignin content, exothermic crystallization was not observed due to disruption of crystalline domain of PCL. The Tg values of the prepared copolymers were found to vary with the weight percent of the PCL in the network.97
3.2 Transacetalization vitrimers
Polyacetals can be formed via acid-catalyzed reaction of an alcohol moiety with aldehyde, accompanied by the release of a molar equivalence of water.98,99 Acetal-based polymers with robust mechanical properties have also been synthesized via “click” addition between a hydroxyl group and vinyl ether in presence of an acid catalyst, without elimination of small molecules.100–102Since acidic conditions can facilitate the cleavage of acetal groups, incorporation of acid in the cross-linked polyacetal networks can help to achieve recyclability and degradability. Songqi and coworkers took advantage of acid-catalyzed acetal exchange to prepare high-performance and recyclable thermosets from the spiro diacetal prepolymer made from lignin-derived vanillin.103 A rigid spiro diacetal, obtained by reacting vanillin with pentaerythritol in the presence of p-toluenesulfonic acid, was modified to degradable thermosets by curing with epichlorohydrin (Scheme 3). The synthesized samples were stable in neutral and basic solutions, but readily degraded in acidic medium. From TGA and tensile tests, thermal stability (Td5% = 278 °C; Td50% = 429 °C) and tensile strength (85 MPa) of the synthesized thermosets were comparable to that of cured bisphenol A epoxy resin (DER331). However, samples from lignin-derived spiro diacetal exhibited higher storage modulus (3131 MPa) and Tg (169 °C from DMA 164 °C from DSC) than DER331 which were 2779 MPa and 164 °C (from DMA) and 157 °C (from DSC), respectively. Recyclability and potential use of lignin-based spiro diacetal thermosets in composites and coating were demonstrated using carbon fiber and glass substrate. Carbon fiber composites and glass coating were prepared using the synthesized thermoset, and its performance was compared with commercial DER331. The tensile strength of the composite prepared with the synthesized product (731 MPa) was higher than that of the commercial epoxy-based composite (661 MPa), while the storage modulus was similar (4.00 GPa and 4.05 GPa, respectively). The recycled carbon fiber composite retained its morphology and thermal–mechanical integrity. The coating on the glass substrate exhibited excellent pencil hardness, flexibility, and solvent resistance and could be removed easily in an acidic medium.
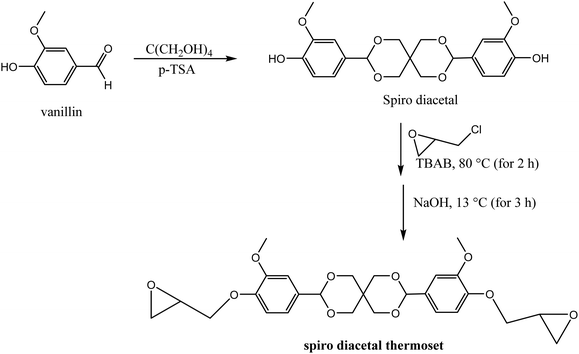 | ||
| Scheme 3 Synthesis of recyclable trans-acetal thermoset from vanillin-derived diacetal.103 | ||
When traces of acid remain in the polymer with acetal linkages, the rate of degradation is accelerated, thus affecting the stability of the network. To avoid this, Moreno and coworkers reported a catalyst-free lignin-based vitrimers with thermally labile acetal bond using softwood kraft lignin (SKL) and poly(ethylene glycol) divinyl ether (PDV).104 In their work, malleable vitrimers with lignin content between 28 and 50% by weight were synthesized by dissolving an appropriate amount of unmodified SKL and PDV in p-dioxane, and then curing the mixture at 110 °C and 150 °C for 6 h and 12 h, respectively. Young's modulus (12.6–2100 MPa), tensile strength (3.3–50.9 MPa), Tg (109–121 °C), and thermal stability of the synthesized samples increased as more lignin was added, which was due to improved cross-linking density and the rigidity of the network at higher lignin contents. The results demonstrated that the mechanical and thermal properties of SKL-PDV vitrimers can be adjusted by varying the amount of lignin. The stress relaxation rates of the samples decreased with decreasing lignin content, where the relaxation times of 77 s, 181 s, 342 s and 2500 s were recorded at 180 °C for samples with 50%, 44%, 37% and 28% of lignin content, respectively. This was attributed to the high number of unreacted OH groups at high lignin content which consequently promoted trans-acetalization reaction. This conclusion was further supported by the observed trend in activation energy that decreased from 204 to 77 kJ mol−1 as SKL content increased from 37 to 50%. The reprocessability via compression molding at 150 °C for 2 h and the potential application of the vitrimers as adhesives were demonstrated using a PDV-SKL sample with 50% SKL. Compared with the virgin sample, the tensile strength (48.1 MPa) of the reprocessed sample did not exhibit any significant change, and no degradation sign was observed from FT-IR and DSC analysis. Furthermore, when two aluminum sheets were bonded with the precured vitrimers, the lap shear stress (6.0 MPa) of the bonded sheets after post-curing was found to be higher than biobased epoxy-vitrimer derived from soybean oil as reported in the literature.105 When applied to the wood, the lap shear stress (2.6 MPa) was comparable to that of other reported lignin-epoxy resins.
3.3 Vitrimers with dynamic imine bonds
Some monomers derived from depolymerization of lignin have received considerable attention for preparation of bio-based vitrimers. Vanillin and its derivatives, such as vanillic acid, eugenol, and vanillyl alcohol, are some of the well-characterized monomers derived from depolymerization of lignin.106–108 Several polymers derived from lignin-based vanillin and its derivatives in combination with fossil based or renewable monomer are reported in the literature.81,109,110Relying on imine chemistry, Geng and coworkers reported bio-based vitrimers by crosslinking lignin-derived vanillin and diethylenetriamine with tris(2-aminoethyl)amine.109 Using 1,4-dibromobutane, a dialdehyde monomer of vanillin (DAV) was synthesized and mixed with the appropriate amount of diethylenetriamine and tris(2-aminoethyl)amine to form a thermally stable polyimine cross-linked networks. The onset decomposition temperature (349–368 °C), DSC-based Tg (48–64 °C), storage modulus (1713–2341 MPa), and tensile strength (47.43–57.1 MPa) of the prepared samples increased as the degree of cross-linking increased. Interestingly, the dynamic nature of the networks was related to imine metathesis, as opposed to the widely acknowledged bond exchange reactions. From the self-healing tests, a scratched sample fully recovered in 2 h at 180 °C, while when two cut pieces of dog-bone shape were realigned at 150 °C for 1 h, a 118.55% and 74.5% recovery in elongation at break and tensile strength, respectively, were achieved. Reprocessability was demonstrated by hot-pressing of small pieces at 150 °C. Even after several cycles of hot pressing, the samples retained their mechanical properties, with some exhibiting slightly higher tensile strength and elongation at break than the original samples. Chemical recycling could be realized in a hot acidic medium. When immersed in hot acidic medium, the vitrimers degraded completely due to the reversible nature of imine bonds. The recycled aldehyde exhibited no structural difference from the original DAV and was re-used to prepare polyschiff vitrimers.
In 2024, Liu and Bernaerts reported lignin-based vitrimers from aldehyde-modified soda lignin via acetalization reaction between dialdehyde and hydroxyl functionalized lignin. The modified lignin (hard segment) was crosslinked with Priamine 1075 (a fatty acid diamine) by hot-pressing at 170 °C via imine chemistry111 to form vitrimers (Scheme 4) with potential application in wood coating. The cross-linking density, storage modulus (800–2200 MPa), Young's modulus (3.4 ± 0.3–1225.4 ± 75.9 MPa), and Tg (16–127 °C) of all the synthesized vitrimers increased with increase in lignin content. However, the tensile stress of vitrimers increased from 1.2 ± 0.2 MPa to 18.4 ± 0.7 MPa when the lignin content increased from 10% to 40%, then decreased to just 8.9 ± 1.4 MPa at 50% lignin content. This sudden decrease was due to dense cross-linkage at higher lignin content that made the material too brittle to be mechanically excellent. Although TGA analysis of the synthesized vitrimers exhibited no stability issue related to reprocessing, increase in lignin content affected the thermal stability of the vitrimers, where a decrease in onset degradation temperature, corresponding to 5% weight loss, was noted (i.e., from 345 °C to 314 °C for vitrimers with 10% to 50% lignin content, respectively). To assess the dynamic nature of the imine bonds, creep recovery, stress relaxation, and reprocessability tests were performed using vitrimers containing 40% lignin (LP-40%). Creep deformation increased with the increase in test temperature, with the vitrimer returning to its original shape below the Tg temperature. Above the Tg, the material assumes a permanent deformation due to a rapid bond exchange that hinders the return to its original shape. On the other hand, the stress relaxation time decreased with temperature (from 170 s to 50 s at 110 °C and 140 °C, respectively) with an Ea of 49.6 kJ mol−1. The topology freezing temperature (58 °C) was lower than the Tg of the vitrimer (78 °C). After three cycles of reprocessing through hot-pressing at 170 °C, the reprocessed samples exhibited identical thermal–mechanical properties with the original sample. Furthermore, synthesized vitrimers were chemically stable in 0.1 M HCl and 2-methyl tetrahydrofuran (2-MeTHF) but degraded in acidified 2-MeTHF. This degradation was attributed to the fact that, as the polymer swells in a polar solvent, H+ from the acid readily enters the network, thus catalyzing the degradation.
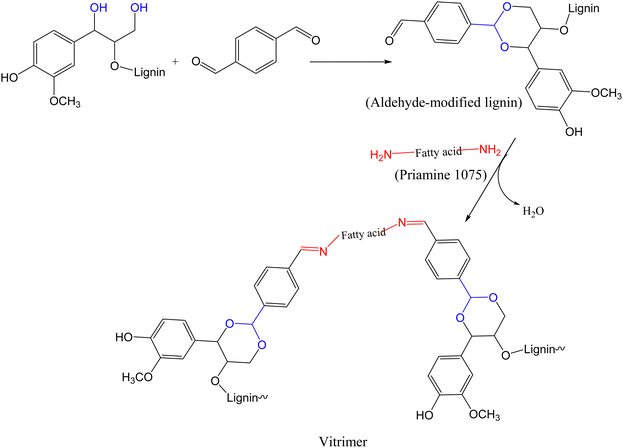 | ||
| Scheme 4 Synthesis of lignin-based vitrimer from aldehyde-modified lignin and Priamine 1075.111 | ||
In a different study, Yu et al. described the synthesis of vanillin-based epoxy (Van-Ep) vitrimer with dynamic imine bonds by curing Van-Ep with isophorone diamine (IPDA).110 First, mono-glycidylated vanillin (Van-Ep) was prepared in a single-step procedure as shown in Scheme 5. The catalyst-free polymer network was synthesized by mixing Van-Ep with IPDA and curing for 2 h at 80 °C and for another 2 h at 100 °C. The synthesized sample exhibited a Tg at 109 °C, initial degradation (Td5%) at 222 °C, storage modulus of 3.56 GPa (at 25 °C), Young's modulus of 2.30 GPa, tensile strength of 65.0 MPa, and reprocessability by hot-pressing at 130 °C. Chemical degradation was demonstrated using a strong hydrochloric acid solution (1 M) at 70 °C, leading to the cleavage of labile imine bonds. Moreover, due to the imine exchange reaction, the vanillin-based vitrimer was able to relax completely above its Tg value, with a relaxation time of 309 s and 50 s at 125 °C and 150 °C, respectively, and an activation energy of 90.25 kJ mol−1.
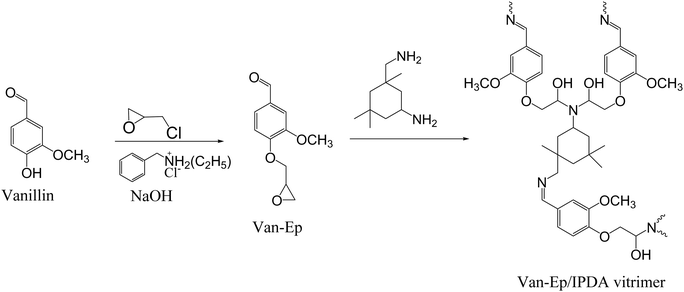 | ||
| Scheme 5 Synthesis path for Van-EP/IPDA vitrimer with dynamic imine bonds.110 | ||
3.4 Transcarbamoylation reactions
Polyurethanes (PU) are among the widely used polymeric materials given their wide application as foams, adhesives, elastomers, and coatings. However, their recyclability has not been properly addressed,112–114 in which polyurethane-based vitrimers could be a solution. Reversibility of the carbamate bond for synthesized of PU vitrimers was first observed by the Urban group in conventional alcohol/isocyanate PU in the presence of a zinc-based catalyst.115 To further develop sustainable polymers, non-isocyanate polyurethanes (NIPUs), also known as polyhydroxyurethanes (PHUs) are being intensively studied.116,117NIPUs are synthesized by polyaddition reaction between polyfunctional carbonates and amines. Only a few studies have reported the preparation and characterization of lignin-based PHUs. Zhao and coworkers reported the first catalyst-free lignin-based PHU vitrimer from the polyaddition of vegetable oil-derived polyamine (Priamine 1074) with bis(6-membered cyclic carbonate), short for BCC, in the presence of enzymatic hydrolysis lignin.114 The thermal stability of the synthesized samples increased with an increase in the lignin content of up to 30% as exhibited by the temperature at the maximum rate of degradation (Tmax) that changed from 443 to 447 °C as the lignin content changed from 0 to 30%. However, the initial degradation temperature T5%, (318–273 °C), associated with the degradation of lignin and cleavage of urethane bonds, decreased as lignin content increased from 0 to 50%. Moreover, increase in hydrogen bonding with increase in lignin content led to an increase in Tg of the synthesized samples (−3.1 to 3.6 °C). Young's modulus (1.7–43.2 MPa), and tensile strength (1.7–11.6 MPa) increased as the lignin content increased from 0 to 50%, while the toughness of the samples increased from 4.9 to 8.2 MJ m−3 (for PHUs from 0 to 30%) and then decreased with increase in lignin content. Thermal reprocessability of cut pieces by hot-pressing was demonstrated at 160 °C. After four rounds of reprocessing, 83.1%, 89.5%, and 71.9% of tensile strength, Young's modulus and tensile toughness, respectively, were retained. The self-healing of cracked samples was observed at 120 °C where 90% recovery was achieved in just 10 s, thanks to the efficient and fast trans-carbamylation bond exchange reaction. Furthermore, shape memory behavior, chemical recyclability in n-butylamine at room temperature, and potential use of synthesized vitrimers in smart packaging and fabricating material were also demonstrated.
Polyurethane-like materials, also known as vinylogous urethane (VU), have been prepared via a condensation reaction between amines and acetoacetates,118 a technique that has been employed to fabricate various advanced materials.119,120 For the first time, Sougrati et al. reported lignin-based VU vitrimers by reacting modified organosolv lignin (OSL) with hexamethylenediamine (HMDA).121 First, modified OSL was obtained by oxyalkylation using a mixture of ethylene carbonate and PEG, and the resulting polyols were converted into polyacetoacetates using tert-butyl acetoacetate (TBAA), as shown in Scheme 6. Five samples of vitrimers with varying lignin content (30–50%) were then synthesized by mixing the polyacetoacetate with HDMA and cured for 24 h at 90 °C followed by post-curing at 150 °C for 30 min. Thermal analysis of the synthesized samples showed that initial degradation, corresponding to 5% weight loss, (Td5%, 244–270 °C) and Tg (−28 to 18 °C) increased with increase in lignin content and the cross-linking density. Though the Td5% slightly increased with PEG chain length, Tg values were not affected by the length of the PEG chain in the networks. The tensile strength (0.2–17.2 MPa) and Young's modulus (0.2–420 MPa) of the synthesized vitrimers correlated positively with lignin content. Whereas no clear trend was observed between stress relaxation times (39 to 488 s) at 130 °C and lignin content, the corresponding Ea for the transamination reaction (51–114 kJ mol−1) increased as the lignin content increased. The potential for chemical recycling was demonstrated using n-octylamine at 100 °C, where the sample dissolved completely. From the self-healing test, the surface-cracked sample exhibited almost 100% recovery after 6 h of heating at 140 °C, demonstrating excellent repairability of the vitrimers.
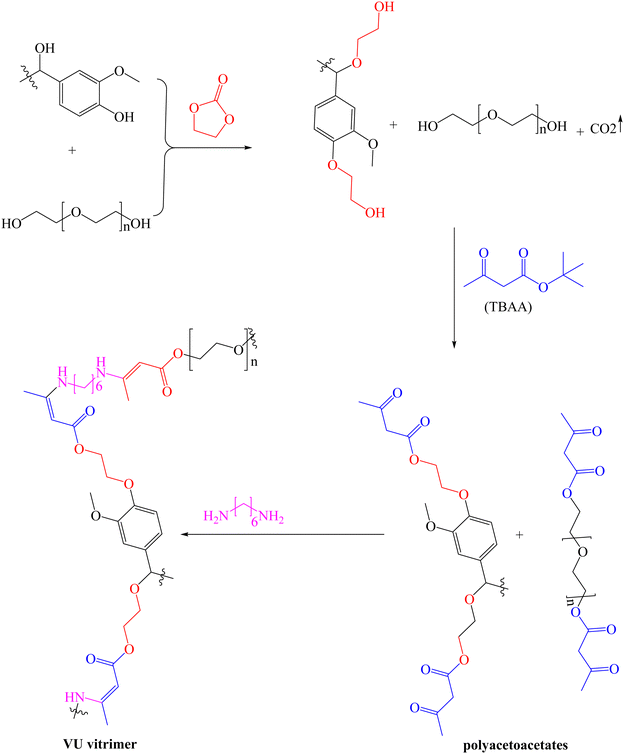 | ||
| Scheme 6 Synthesis of polyacetoacetates from organosolv lignin.121 | ||
4. Conclusions
This review aims to give an overview of the growing area of lignin-based vitrimers. Together with numerous vitrimer chemistries, the development of various lignin-based building blocks enables the synthesis of cross-linked materials possessing thermal–mechanical properties of thermosets at room temperature but can be reprocessed, recycled and self-healed at elevated temperatures. This places lignin as a viable candidate for the development of a sustainable circular material, which acts as a substitute for fossil-based feedstock.As depicted in this review, the first conclusion that can be drawn from the categorization of lignin-based vitrimers according to their vitrimeric chemistry is on the most common lignin modification strategies. Lignin epoxidation using epichlorohydrin and carboxylation (using ozone and anhydrides) has attracted much attention thanks to dominance of transesterification vitrimers and the ease of modification. However, there is a need to balance the sustainability of these modification approaches since ozone and epichlorohydrin are potential carcinogens and toxic.
Furthermore, this review highlights the potential use of lignin-based vitrimers as recoverable adhesives, elastomers, foam, coatings, and composite matrices (Table 6). However, it is apparent that the properties and potential usage of vitrimers are highly dependent on an array of parameters such as the nature of the starting material, activation energy of the dynamic covalent linkages within the network, crosslinking chemistry, and nature of additives used in vitrimeric composite formation.122 Depending on the intended application, tuning these parameters is crucial when preparing vitrimers. For instance, multi-cycle reprocessing and mechanical recycling are key in the thermoforming of composites. In such a case, considering a combination of material of sufficient mechanical strength, alongside catalysts less likely to accelerate degradation of dynamic bonds, will be of paramount importance. Moreover, in applications where reprocessability is needed, such as injection molding, dynamic bonds with low activation energy, such as boronate esters where exchange can happen without a catalyst,123 would be suitable. Vitrimers intended for usage at higher temperatures would require dynamic crosslinkages that are more stable and resistant to reaction even at elevated temperatures, such as the Si–O–Ph linkage in the benzoxanime resin [poly(P-mdes)] vitrimeric network.124 Beyond substituting fossil-based polymers, lignin-based vitrimers should be considered as one of the next-generation materials for use in 3D/4D printing and various demanding applications like those in biomedical fields.
| Vitrimer | Thermal-mechanical | E a (kJ mol−1) | Recycling | Application | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | Curing conditions | Dynamic covalent chemistry | Strength (MPa) | Storage modulus (MPa) | T g (°C) | Onset degradation temperature (°C) | Physical | Chemical | |||
| a Determined from DSC curves. b Determined by DMA from Tan δ curves. c Temperature at 5% weight loss. d Temperature at 10% weight loss. e Obtained from stress relaxation experiments. | |||||||||||
| Ozonated kraft lignin, epoxidized sebacic acid | Zn(acac)2 catalyst 150 °C, 190 °C | Transesterification | 5.1–12.9 | >103 | 95–133b | 222–274c | — | Hot pressing at 190 °C | — | Adhesive | 58 |
| Epoxidized kraft lignin, PEG-Bis (CMC) | Zinc acetate catalyst. 120 °C | Transesterification | 1.45–4.52 | — | 191a | 280–315c | — | Hot-pressing at 150 °C | — | Adhesive | 77 |
| Light-controlled remolding | |||||||||||
| Ozonated enzymatic lignin, carboxylated enzymatic lignin | Zinc acetate catalyst 120 °C | Transesterification | 48–68 | 1250–4000 | 100.01–102.52a | 262.54–286.04c | 18.12e | Hot-pressing at 150 °C & 4 MPa | Ethylene glycol at 190 °C | Adhesive, coating, rubber bonding | 78 |
| 100.51–138.49b | |||||||||||
| Lignin-derived vanillic acid, poly(ethylene glycol)diglycidyl ether | Zn(acac)2 catalyst 110 °C | Transesterification | — | 70.6–208 | 36.5–75.9a | 260.0–283.5c | 54.42e | — | — | — | 79 |
| 39.6–51.7b | |||||||||||
| 4-Methylcatechol, vanillyl alcohol, & a mixture of fatty acids dimers and trimers | Zn(acac)2 catalyst 65 °C, 120 °C, 160 °C | Transesterification | 2.7–25.1 | >103 | 11–23a | 300–354c | 24.3–59.5e | Melt-molding at 100 °C | — | Adhesive | 81 |
| 34–38b | |||||||||||
| Glycidyl etherified enzymatic hydrolysis lignin, DGEBA, & dodecanedioic acid | Zn(acac)2 catalyst 120 °C, 160 °C | Transesterification | 4.9–39.5 | 502.6–1004.6 | 23.1–46.9a | 290.1–328.3d | Hot-pressing at 190 °C | — | — | 82 | |
| 34.5–77.3b | |||||||||||
| Epoxidized enzymatic hydrolysis lignin, sebacic acid | Zn(acac)2 catalyst 120 °C, 160 °C | Transesterification | 20.79–39.82 | 920–1220 | 40.4–66.8a | 276.35–297.54c | Hot-pressing at 160 °C & 5 MPa | — | — | 85 | |
| 72.59–103.47b | |||||||||||
| Epoxidized enzymatic lignin, carboxylated enzymatic lignin | Zinc acetate catalyst 150 °C, 170 °C | Transesterification | — | — | — | — | 97.21e | — | — | Road asphalt | 86 |
| Kraft lignin, citric acid, PEG | DMAP catalyst 110 °C | Transesterification | 1.8–3.7 | 70–190 (at 20 °C) | 4.4–10.2a | — | — | — | Microwave-heated alkaline soluton at 160 °C | — | 92 |
| Organosolv lignin, boronic acid-modified polycaprolactone | MgSO4 dehydrating agent, 65 °C | Transesterification | — | — | −60 to ∼50a | ≈300–370c | — | — | — | 97 | |
| Lignin-derived vanillin, epichlorohydrin | Tetrabutyl ammonium bromide catalyst 80 °C | Transacetalization | 85 | 3131 | 164a | 278c | — | — | — | Composite formation, glass coating | 103 |
| 169b | |||||||||||
| Kraft lignin, poly(ethyelene glycol) divinyl ether | 110 °C, 150 °C | Transacetalization | 3.3–50.9 | >103 | 109–121a | 202–224c | 77–204e | Compression molding at 150 °C | — | Adhesive | 104 |
| Lignin-derived vanillin, diethylenetriamine, tris(2-aminoethyl)amine | 50 °C | Imine metathesis | 47.43–57.1 | 1713–2341 | 48–64a | 349–368c | — | Hot-pressing at 150 °C | Hot acidic medium | — | 109 |
| Aldehyde-modified soda lignin, fatty acid diamine (Priamine 1075) | Hot-pressed at 170 °C | Transamination | 1.2–18.4 | 800–2200 | 16–127a | 314–345c | 49.6e | Hot-pressing at 170 °C | — | Wood coating | 111 |
| Epoxidized vanillin, isophorone diamine | 80 °C, 100 °C | Transamination | 65.0 | 3560 | 109a | 222c | 90.25e | Hot-pressing at 130 °C | 1 M HCl at 70 °C | — | 110 |
| 121b | |||||||||||
| Enzymatic hydrolysis lignin, Priamine 1074 | Hot-pressed at 160 °C and 4 MPa | Transcarbamylation | 1.7–11.6 | — | −3.1 to 3.6a | 273–318c | — | Hot-pressing at 160 °C | n-Butylamine at room temperature | Smart packaging, material fabrication | 114 |
| Oxyalkylated organosolv lignin, hexamethylenediamine | 90 °C, 150 °C | Transamination | 0.2–17.2 | >900 | −28–18a | 244–270c | 51–114e | n-Octylamine at 100 °C | 121 | ||
Lastly, since lignin-based vitrimers demonstrate the ability to meet the market requirements, incorporating abundance, price, and rigidity help to place lignin as a key alternative feedstock to lower the carbon footprint and contribute towards a circular economy.
Author contributions
Peter K. Karoki: wrote the manuscript. Shuyang Zhang: reviewed and provided critical feedback on the initial draft manuscript. Yunqiao Pu: reviewed and provided critical feedback on the initial draft manuscript. Arthur J. Ragauskas: critical review and overall supervision.Conflicts of interest
The authors have no known competing interests to declare.Acknowledgements
The authors wish to thank the Southeastern Sun Grant Center for funding “Lignin Based Polyester Vitrimers” which supported this project in part. YP and AJR also wish to acknowledge the support of the Center for Bioenergy Innovation (CBI), U.S. Department of Energy, Office of Science, Biological and Environmental Research Program under Award Number ERKP886.References
- A. R. Rennie, Mechanical Properties and Testing of Polymers, in Thermoplastics and Thermosets, ed. Swallowe M. G., Springer, Dordrecht, 1999, vol. 3 DOI:10.1007/978-94-015-9231-4_53.
- E. Morici and N. T. Dintcheva, Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity, Polymers, 2022, 14(19), 4153, DOI:10.3390/polym14194153.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Covalent Adaptable Networks (CANs): A Unique Paradigm in Crosslinked Polymers, Macromolecules, 2010, 43(6), 2643–2653, DOI:10.1021/ma902596s.
- N. Van Herck, D. Maes, K. Unal, M. Guerre, J. M. Winne and F. E. Du Prez, Covalent Adaptable Networks with Tunable Exchange Rates Based on Reversible Thiol-yne Cross-Linking, Angew. Chem., Int. Ed., 2020, 59(9), 3609–3617, DOI:10.1002/anie.201912902.
- S. Yoon, J. H. Choi, B. J. Sung, J. Bang and T. A. Kim, Mechanochromic and thermally reprocessable thermosets for autonomic damage reporting and self-healing coatings, NPG Asia Mater., 2022, 14(1), 61, DOI:10.1038/s41427-022-00406-3.
- W. Denissen, J. M. Winne and F. E. Du Prez, Vitrimers: permanent organic networks with glass-like fluidity, Chem. Sci., 2016, 7(1), 30–38, 10.1039/c5sc02223a.
- B. R. Elling and W. R. Dichtel, Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable?, ACS Cent. Sci., 2020, 6(9), 1488–1496, DOI:10.1021/acscentsci.0c00567.
- T. Maeda, H. Otsuka and A. Takahara, Dynamic covalent polymers: Reorganizable polymers with dynamic covalent bonds, Prog. Polym. Sci., 2009, 34(7), 581–604, DOI:10.1016/j.progpolymsci.2009.03.001.
- X. Chen, A. D. Matheus, O. Kanji, M. Ajit, S. Hongbin, R. N. Steven, S. Kevin and W. Fred, A Thermally Re-mendable Cross-Linked Polymeric Material, Science, 2002, 295(5560), 1698–1702, DOI:10.1126/science.1065879.
- T. F. Scott, A. D. Schneider, W. D. Cook and C. N. Bowman, Photoinduced plasticity in cross-linked polymers, Science, 2005, 308(5728), 1615–1617, DOI:10.1126/science.1110505.
- T. Vidil and A. Llevot, Fully Biobased Vitrimers: Future Direction toward Sustainable Cross-Linked Polymers, Macromol. Chem. Phys., 2022, 223(13) DOI:10.1002/macp.202100494.
- M. A. Lucherelli, A. Duval and L. Avérous, Biobased vitrimers: Towards sustainable and adaptable performing polymer materials, Prog. Polym. Sci., 2022, 127, 101515, DOI:10.1016/j.progpolymsci.2022.101515.
- N. Lu, Q. Li, S. Ma, B. Wang, X. Xu, S. Wang, J. Ye, J. Qiu and J. Zhu, Scalable and facile synthesis of acetal covalent adaptable networks with readily adjustable properties, Eur. Polym. J., 2021, 147, 110291, DOI:10.1016/j.eurpolymj.2021.110291.
- A. Chao, I. Negulescu and D. Zhang, Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent, Macromolecules, 2016, 49(17), 6277–6284, DOI:10.1021/acs.macromol.6b01443.
- C. M. Montarnal, F. Tournilhac and L. Leibler, Silica-Like Malleable Materials from Permanent Organic Networks, Science, 2011, 334(6058), 965–968, DOI:10.1126/science.1212648.
- W. Alabiso and S. Schlogl, The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications, Polymers, 2020, 12(8), 1660, DOI:10.3390/polym12081660.
- K. Balaji, P. Andrea, P. W. Prakash, K. Indresh, H. B. Wolfgang and R. Sravendra, Vitrimers based on bio-derived chemicals: Overview and future prospects, Chem. Eng. J., 2022, 433, 133261, DOI:10.1016/j.cej.2021.133261.
- Z. Wang, M. S. Ganewatta and C. Tang, Sustainable polymers from biomass: Bridging chemistry with materials and processing, Prog. Polym. Sci., 2020, 101, 101197, DOI:10.1016/j.progpolymsci.2019.101197.
- M. Adsul, D. K. Tuli, P. K. Annamalai, D. Depan and S. Shankar, Polymers from Biomass: Characterization, Modification, Degradation, and Applications, Int. J. Polym. Sci., 2016, 2016, 1–2, DOI:10.1155/2016/1857297.
- R. M. O'Dea, J. A. Willie and T. H. Epps, 100th Anniversary of Macromolecular Science Viewpoint: Polymers from Lignocellulosic Biomass. Current Challenges and Future Opportunities, ACS Macro Lett., 2020, 9(4), 476–493, DOI:10.1021/acsmacrolett.0c00024.
- S. Rana, M. Solanki, N. G. Sahoo and B. Krishnakumar, Bio-Vitrimers for Sustainable Circular Bio-Economy, Polymers, 2022, 14(20), 4338, DOI:10.3390/polym14204338.
- K. L. Chong, J. C. Lai, R. A. Rahman, N. Adrus, Z. H. Al-Saffar, A. Hassan, T. H. Lim and M. U. Wahit, A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils, Ind. Crops Prod., 2022, 189, 115857, DOI:10.1016/j.indcrop.2022.115857.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Lignin valorization: improving lignin processing in the biorefinery, Science, 2014, 344(6185), 1246843, DOI:10.1126/science.1246843.
- A. Zoghlami and G. Paes, Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis, Front. Chem., 2019, 7, 874, DOI:10.3389/fchem.2019.00874.
- M. Naebe and Y. Zhang, Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber, ACS Sustainable Chem. Eng., 2021, 9(4), 1427–1442, DOI:10.1021/acssuschemeng.0c06998.
- J. C. del Río, J. Rencoret, A. Gutiérrez, T. Elder, H. Kim and J. Ralph, Lignin Monomers from beyond the Canonical Monolignol Biosynthetic Pathway: Another Brick in the Wall, ACS Sustainable Chem. Eng., 2020, 8(13), 4997–5012, DOI:10.1021/acssuschemeng.0c01109.
- Y. Pu, S. Cao and A. J. Ragauskas, Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization, Energy Environ. Sci., 2011, 4(9), 3154–3166, 10.1039/c1ee01201k.
- J. M. Lang, U. M. Shrestha and M. Dadmun, The Effect of Plant Source on the Properties of Lignin-Based Polyurethanes, Front. Energy Res., 2018, 6 DOI:10.3389/fenrg.2018.00004.
- J. M. Jardim, P. W. Hart, L. Lucia and H. Jameel, Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery, Polymers, 2020, 12(8), 1795, DOI:10.3390/polym12081795.
- M. J. Suota, T. A. da Silva, S. F. Zawadzki, G. L. Sassaki, F. A. Hansel, M. Paleologou and L. P. Ramos, Chemical and structural characterization of hardwood and softwood LignoForce™ lignins, Ind. Crops Prod., 2021, 173, 114138, DOI:10.1016/j.indcrop.2021.114138.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. Bruijnincx and B. M. Weckhuysen, Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis, Angew. Chem., Int. Ed., 2016, 55(29), 8164–8215, DOI:10.1002/anie.201510351.
- X. Liu, F. P. Bouxin, J. Fan, V. L. Budarin, C. Hu and J. H. Clark, Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review, ChemSusChem, 2020, 13(17), 4296–4317, DOI:10.1002/cssc.202001213.
- S. K. Singh and P. L. Dhepe, Isolation of lignin by organosolv process from different varieties of rice husk: Understanding their physical and chemical properties, Bioresour. Technol., 2016, 221, 310–317, DOI:10.1016/j.biortech.2016.09.042.
- H. Chen, Lignocellulose biorefinery feedstock engineering, Lignocellulose Biorefinery Engineering, 2015, pp. 37–86 DOI:10.1016/b978-0-08-100135-6.00003-x.
- S. Bhagia and A. J. Ragauskas, Preserving Aryl Ether Linkages and Higher Yields of Isolated Lignin through Biomass Fibrillation, ACS Sustainable Chem. Eng., 2019, 8(1), 34–37, DOI:10.1021/acssuschemeng.9b06390.
- A. Tolbert, H. Akinosho, R. Khunsupat, A. K. Naskar and A. J. Ragauskas, Characterization and analysis of the molecular weight of lignin for biorefining studies, Biofuels, Bioprod. Biorefin., 2014, 8(6), 836–856, DOI:10.1002/bbb.1500.
- F. S. Chakar and A. J. Ragauskas, Review of current and future softwood kraft lignin process chemistry, Ind. Crops Prod., 2004, 20(2), 131–141, DOI:10.1016/j.indcrop.2004.04.016.
- T. Aro and P. Fatehi, Production and Application of Lignosulfonates and Sulfonated Lignin, ChemSusChem, 2017, 10(9), 1861–1877, DOI:10.1002/cssc.201700082.
- A. A. Vaidya, K. D. Murton, D. A. Smith and G. Dedual, A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose, Biomass Convers. Biorefin., 2022, 12(11), 5427–5442, DOI:10.1007/s13399-022-02373-9.
- J. Liu, X. Li, M. Li and Y. Zheng, Lignin biorefinery: Lignin source, isolation, characterization, and bioconversion, Adv. Bioenergy, 2022, 211–270, DOI:10.1016/bs.aibe.2022.05.004.
- J. Xu, C. Li, L. Dai, C. Xu, Y. Zhong, F. Yu and C. Si, Biomass Fractionation and Lignin Fractionation towards Lignin Valorization, ChemSusChem, 2020, 13(17), 4284–4295, DOI:10.1002/cssc.202001491.
- P. Kumar, D. M. Barrett, M. J. Delwiche and P. Stroeve, Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production, Ind. Eng. Chem. Res., 2009, 48, 3713–3729, DOI:10.1021/ie801542g.
- Z. M. Zhao, X. Meng, B. Scheidemantle, Y. Pu, Z. H. Liu, B. Z. Li, C. E. Wyman, C. M. Cai and A. J. Ragauskas, Cosolvent enhanced lignocellulosic fractionation tailoring lignin chemistry and enhancing lignin bioconversion, Bioresour. Technol., 2022, 347, 126367, DOI:10.1016/j.biortech.2021.126367.
- R. El Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrahi and A. Ragauskas, Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus, Polym. Degrad. Stab., 2009, 94(10), 1632–1638, DOI:10.1016/j.polymdegradstab.2009.07.007.
- A. Guerra, I. Filpponen, L. A. Lucia and D. S. Argyropoulos, Comparative Evaluation of Three Lignin Isolation Protocols for Various Wood Species, J. Agric. Food Chem., 2006, 54(26), 9696–9705, DOI:10.1021/jf062433c.
- A. De Santi, S. Monti, G. Barcaro, Z. Zhang, K. Barta and P. J. Deuss, New Mechanistic Insights into the Lignin beta-O-4 Linkage Acidolysis with Ethylene Glycol Stabilization Aided by Multilevel Computational Chemistry, ACS Sustainable Chem. Eng., 2021, 9(5), 2388–2399, DOI:10.1021/acssuschemeng.0c08901.
- W. G. Glasser, About Making Lignin Great Again-Some Lessons From the Past, Front. Chem., 2019, 7, 565, DOI:10.3389/fchem.2019.00565.
- C. Ma, T. H. Kim, K. Liu, M. G. Ma, S. E. Choi and C. Si, Multifunctional Lignin-Based Composite Materials for Emerging Applications, Front. Bioeng. Biotechnol., 2021, 9, 708976, DOI:10.3389/fbioe.2021.708976.
- Y. Lu, Y.-C. Lu, H.-Q. Hu, F.-J. Xie, X.-Y. Wei and X. Fan, Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods, J. Spectrosc., 2017, 2017, 1–15, DOI:10.1155/2017/8951658.
- Y. Matsushita, Conversion of technical lignins to functional materials with retained polymeric properties, J. Wood Sci., 2015, 61(3), 230–250, DOI:10.1007/s10086-015-1470-2.
- N. Lee, Y. T. Kim and J. Lee, Recent Advances in Renewable Polymer Production from Lignin-Derived Aldehydes, Polymers, 2021, 13(3), 364, DOI:10.3390/polym13030364.
- S. M. Suh, S. Jambu, M. T. Chin and T. Diao, Selective Cleavage of Lignin Model Compounds via a Reverse Biosynthesis Mechanism, Org. Lett., 2023, 25(26), 4792–4796, DOI:10.1021/acs.orglett.3c01416.
- D. Kai, M. J. Tan, P. L. Chee, Y. K. Chua, Y. L. Yap and X. J. Loh, Towards lignin-based functional materials in a sustainable world, Green Chem., 2016, 18(5), 1175–1200, 10.1039/c5gc02616d.
- K. A. Y. Koivu, H. Sadeghifar, P. A. Nousiainen, D. S. Argyropoulos and J. Sipilä, Effect of Fatty Acid Esterification on the Thermal Properties of Softwood Kraft Lignin, ACS Sustainable Chem. Eng., 2016, 4(10), 5238–5247, DOI:10.1021/acssuschemeng.6b01048.
- S. H. Hong, J. H. Park, O. Y. Kim and S. H. Hwang, Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance, Polymers, 2021, 13(4), 667, DOI:10.3390/polym13040667.
- J. Sun, C. Wang, L. P. Stubbs and C. He, Carboxylated Lignin as an Effective Cohardener for Enhancing Strength and Toughness of Epoxy, Macromol. Mater. Eng., 2017, 302(12), 1700341, DOI:10.1002/mame.201700341.
- T. L. Eberhardt, Y. Zhang, H. Wang, Q. Gu and H. Pan, Preparation of carboxylated lignin-based epoxy resin with excellent mechanical properties, Eur. Polym. J., 2021, 150, 110389, DOI:10.1016/j.eurpolymj.2021.110389.
- S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives, Green Chem., 2018, 20(13), 2995–3000, 10.1039/c8gc01299g.
- J. R. Silverman, A. M. Danby and B. Subramaniam, Intensified ozonolysis of lignins in a spray reactor: insights into product yields and lignin structure, React. Chem. Eng., 2019, 4(8), 1421–1430, 10.1039/c9re00098d.
- M. B. Figueirêdo, F. W. Keij, A. Hommes, P. J. Deuss, R. H. Venderbosch, J. Yue and H. J. Heeres, Efficient Depolymerization of Lignin to Biobased Chemicals Using a Two-Step Approach Involving Ozonation in a Continuous Flow Microreactor Followed by Catalytic Hydrotreatment, ACS Sustainable Chem. Eng., 2019, 7(22), 18384–18394, DOI:10.1021/acssuschemeng.9b04020.
- W. Ruiqi, B. Zhou and Z. Wang, Study on the Preparation and Application of Lignin-Derived Polycarboxylic Acids, J. Chem., 2019, 2019, 1–8, DOI:10.1155/2019/5493745.
- M. B. Figueirêdo, P. J. Deuss, R. H. Venderbosch and H. J. Heeres, Valorization of Pyrolysis Liquids: Ozonation of the Pyrolytic Lignin Fraction and Model Components, ACS Sustainable Chem. Eng., 2019, 7(5), 4755–4765, DOI:10.1021/acssuschemeng.8b04856.
- H. Wang, L. Zhao, J. Ren and B. He, Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility, Processes, 2022, 10(3), 559, DOI:10.3390/pr10030559.
- A. Salanti, L. Zoia and M. Orlandi, Chemical modifications of lignin for the preparation of macromers containing cyclic carbonates, Green Chem., 2016, 18(14), 4063–4072, 10.1039/c6gc01028h.
- J. R. Gouveia, G. E. S. Garcia, L. D. Antonino, L. B. Tavares and D. J. Dos Santos, Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive, Molecules, 2020, 25(11), 2513, DOI:10.3390/molecules25112513.
- H. Pan, G. Sun and T. Zhao, Synthesis and characterization of aminated lignin, Int. J. Biol. Macromol., 2013, 59, 221–226, DOI:10.1016/j.ijbiomac.2013.04.049.
- L. Y. Liu, X. Wan, S. Chen, P. Boonthamrongkit, M. Sipponen and S. Renneckar, Solventless Amination of Lignin and Natural Phenolics using 2-Oxazolidinone, ChemSusChem, 2023, 16(15), e202300276, DOI:10.1002/cssc.202300276.
- L. An, C. Si, J. H. Bae, H. Jeong and Y. S. Kim, One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution, Int. J. Biol. Macromol., 2020, 159, 222–230, DOI:10.1016/j.ijbiomac.2020.05.072.
- M. W. Ott, C. Dietz, S. Trosien, S. Mehlhase, M. J. Bitsch, M. Nau, T. Meckel, A. Geissler, G. Siegert, J. Huong, B. Hertel, R. W. Stark and M. Biesalski, Co-curing of epoxy resins with aminated lignins: insights into the role of lignin homo crosslinking during lignin amination on the elastic properties, Holzforschung, 2021, 75(4), 390–398, DOI:10.1515/hf-2020-0060.
- X. Meng, B. Scheidemantle, M. Li, Y. Y. Wang, X. Zhao, M. Toro-Gonzalez, P. Singh, Y. Pu, C. E. Wyman, S. Ozcan, C. M. Cai and A. J. Ragauskas, Synthesis, Characterization, and Utilization of a Lignin-Based Adsorbent for Effective Removal of Azo Dye from Aqueous Solution, ACS Omega, 2020, 5(6), 2865–2877, DOI:10.1021/acsomega.9b03717.
- G.-J. Jiao, P. Peng, S.-L. Sun, Z.-C. Geng and D. She, Amination of biorefinery technical lignin by Mannich reaction for preparing highly efficient nitrogen fertilizer, Int. J. Biol. Macromol., 2019, 127, 544–554, DOI:10.1016/j.ijbiomac.2019.01.076.
- A. Sharma, P. Kaur, G. Singh and S. K. Arya, Economical concerns of lignin in the energy sector, Cleaner Eng. Technol., 2021, 4, 100258, DOI:10.1016/j.clet.2021.100258.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, A concise review of current lignin production, applications, products and their environmental impact, Ind. Crops Prod., 2019, 139, 111526, DOI:10.1016/j.indcrop.2019.111526.
- B. Jiang, H. Jiao, X. Guo, G. Chen, J. Guo, W. Wu, Y. Jin, G. Cao and Z. Liang, Lignin-Based Materials for Additive Manufacturing: Chemistry, Processing, Structures, Properties, and Applications, Adv. Sci., 2023, 10(9), e2206055, DOI:10.1002/advs.202206055.
- A. Adjaoud, L. Puchot, C. E. Federico, R. Das and P. Verge, Lignin-based benzoxazines: A tunable key-precursor for the design of hydrophobic coatings, fire resistant materials and catalyst-free vitrimers, Chem. Eng. J., 2023, 453, 139895, DOI:10.1016/j.cej.2022.139895.
- S. G. Prolongo, G. del Rosario and A. Ureña, Comparative study on the adhesive properties of different epoxy resins, Int. J. Adhes. Adhes., 2006, 26(3), 125–132, DOI:10.1016/j.ijadhadh.2005.02.004.
- Y. Zheng, T. Liu, H. He, Z. Lv, J. Xu, D. Ding, L. Dai, Z. Huang and C. Si, Lignin-based epoxy composite vitrimers with light-controlled remoldability, Adv. Compos. Hybrid Mater., 2023, 6(1) DOI:10.1007/s42114-023-00633-4.
- L. Du, X. Jin, G. Qian, W. Yang, L. Su, Y. Ma, S. Ren and S. Li, Lignin-based vitrimer for circulation in plastics, coatings, and adhesives with tough mechanical properties, catalyst-free and good chemical solvent resistance, Ind. Crops Prod., 2022, 187, 115439, DOI:10.1016/j.indcrop.2022.115439.
- A. More, T. Elder, N. Pajer, D. S. Argyropoulos and Z. Jiang, Novel and Integrated Process for the Valorization of Kraft Lignin to Produce Lignin-Containing Vitrimers, ACS Omega, 2023, 8(1), 1097–1108, DOI:10.1021/acsomega.2c06445.
- C. Hao, T. Liu, S. Zhang, L. Brown, R. Li, J. Xin, T. Zhong, L. Jiang and J. Zhang, A High-Lignin-Content, Removable, and Glycol-Assisted Repairable Coating Based on Dynamic Covalent Bonds, ChemSusChem, 2019, 12(5), 1049–1058, DOI:10.1002/cssc.201802615.
- S. Zhao and M. M. Abu-Omar, Catechol-Mediated Glycidylation toward Epoxy Vitrimers/Polymers with Tunable Properties, Macromolecules, 2019, 52(10), 3646–3654, DOI:10.1021/acs.macromol.9b00334.
- B. Xue, R. Tang, D. Xue, Y. Guan, Y. Sun, W. Zhao, J. Tan and X. Li, Sustainable alternative for bisphenol A epoxy resin high-performance and recyclable lignin-based epoxy vitrimers, Ind. Crops Prod., 2021, 168, 113583, DOI:10.1016/j.indcrop.2021.113583.
- E. D. Hernandez, A. W. Bassett, J. M. Sadler, J. J. La Scala and J. F. Stanzione, Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol, ACS Sustainable Chem. Eng., 2016, 4(8), 4328–4339, DOI:10.1021/acssuschemeng.6b00835.
- D. J. van de Pas and K. M. Torr, Biobased Epoxy Resins from Deconstructed Native Softwood Lignin, Biomacromolecules, 2017, 18(8), 2640–2648, DOI:10.1021/acs.biomac.7b00767.
- R. Tang, B. Xue, J. Tan, Y. Guan, J. Wen, X. Li and W. Zhao, Regulating Lignin-Based Epoxy Vitrimer Performance by Fine-Tuning the Lignin Structure, ACS Appl. Polym. Mater., 2022, 4(2), 1117–1125, DOI:10.1021/acsapm.1c01541.
- P. Song, L. Du, J. Pang, G. Jiang, J. Shen, Y. Ma, S. Ren and S. Li, Preparation and properties of lignin-based vitrimer system containing dynamic covalent bonds for reusable and recyclable epoxy asphalt, Ind. Crops Prod., 2023, 197, 116498, DOI:10.1016/j.indcrop.2023.116498.
- M. Li, X. Chen, P. Cong, C. Luo, L. Zhu, H. Li, Y. Zhang, M. Chao and L. Yan, Facile synthesis of polyethylene-modified asphalt by chain end-functionalization, Compos. Commun., 2022, 30, 101088, DOI:10.1016/j.coco.2022.101088.
- Z. Zhang, Z. Huang, W. Zhou, R. Xu, J. Xu and Y. Liu, Study on synthesis and road performance of light-colored resin modified asphalt. IOP Conf. Ser.: Earth Environ. Sci., 2021, vol. 770, p. 012003 DOI:10.1088/1755-1315/770/1/012003.
- M. A. Vargas, M. A. Vargas, A. Sánchez-Sólis and O. Manero, Asphalt/polyethylene blends: Rheological properties, microstructure and viscosity modeling, Constr. Build. Mater., 2013, 45, 243–250, DOI:10.1016/j.conbuildmat.2013.03.064.
- R. Liu, A. Smeds, L. Wang, A. Pranovich, J. Hemming, S. Willför, H. Zhang and C. Xu, Fractionation of Lignin with Decreased Heterogeneity: Based on a Detailed Characteristics Study of Sequentially Extracted Softwood Kraft Lignin, ACS Sustainable Chem. Eng., 2021, 9(41), 13862–13873, DOI:10.1021/acssuschemeng.1c04725.
- H. Sadeghifar and A. Ragauskas, Perspective on Technical Lignin Fractionation, ACS Sustainable Chem. Eng., 2020, 8(22), 8086–8101, DOI:10.1021/acssuschemeng.0c01348.
- Y. Xu, K. Odelius and M. Hakkarainen, Recyclable and Flexible Polyester Thermosets Derived from Microwave-Processed Lignin, ACS Appl. Polym. Mater., 2020, 2(5), 1917–1924, DOI:10.1021/acsapm.0c00130.
- M. Gosecki and M. Gosecka, Boronic Acid Esters and Anhydrates as Dynamic Cross-Links in Vitrimers, Polymers, 2022, 14(4), 842, DOI:10.3390/polym14040842.
- A. Zych, J. Tellers, L. Bertolacci, L. Ceseracciu, L. Marini, G. Mancini and A. Athanassiou, Biobased, Biodegradable, Self-Healing Boronic Ester Vitrimers from Epoxidized Soybean Oil Acrylate, ACS Appl. Polym. Mater., 2020, 3(2), 1135–1144, DOI:10.1021/acsapm.0c01335.
- X. Zhang, S. Wang, Z. Jiang, Y. Li and X. Jing, Boronic Ester Based Vitrimers with Enhanced Stability via Internal Boron-Nitrogen Coordination, J. Am. Chem. Soc., 2020, 142(52), 21852–21860, DOI:10.1021/jacs.0c10244.
- A. L. Korich, K. M. Clarke, D. Wallace and P. M. Iovine, Chemical Modification of a Lignin Model Polymer via Arylboronate Ester Formation under Mild Reaction Conditions, Macromolecules, 2009, 42(16), 5906–5908, DOI:10.1021/ma901146b.
- A. L. Korich, A. B. Fleming, A. R. Walker, J. Wang, C. Tang and P. M. Iovine, Chemical modification of organosolv lignin using boronic acid-containing reagents, Polymer, 2012, 53(1), 87–93, DOI:10.1016/j.polymer.2011.10.062.
- W. Yanmei, H. Morinaga, A. Sudo and T. Endo, Synthesis of amphiphilic polyacetal by polycondensation of aldehyde and polyethylene glycol as an acid-labile polymer for controlled release of aldehyde, J. Polym. Sci., Part A: Polym. Chem., 2011, 49(3), 596–602, DOI:10.1002/pola.24425.
- L. Na, J. Vignolle, J.-M. Vincent, F. Robert, Y. Landais, H. Cramail and D. Taton, One-Pot Synthesis and PEGylation of Hyperbranched Polyacetals with a Degree of Branching of 100%, Macromolecules, 2014, 47(5), 1532–1542, DOI:10.1021/ma4026509.
- C. Mangold, C. Dingels, B. Obermeier, H. Frey and F. Wurm, PEG-based Multifunctional Polyethers with Highly Reactive Vinyl-Ether Side Chains for Click-Type Functionalization, Macromolecules, 2011, 44(16), 6326–6334, DOI:10.1021/ma200898n.
- Q. Li, S. Ma, P. Li, B. Wang, Z. Yu, H. Feng, Y. Liu and J. Zhu, Fast Reprocessing of Acetal Covalent Adaptable Networks with High Performance Enabled by Neighboring Group Participation, Macromolecules, 2021, 54(18), 8423–8434, DOI:10.1021/acs.macromol.1c01046.
- R. Tomlinson, M. Klee, S. Garrett, J. Heller, R. Duncan and S. Brocchini, Pendent Chain Functionalized Polyacetals That Display pH-Dependent Degradation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Platform for the Development of Novel Polymer Therapeutics, Macromeolecules, 2002, 35(2), 473–480, DOI:10.1021/ma0108867.
A Platform for the Development of Novel Polymer Therapeutics, Macromeolecules, 2002, 35(2), 473–480, DOI:10.1021/ma0108867. - M. Songqi, J. Wei, Z. Jia, T. Yu, W. Yuan, Q. Li, S. Wang, S. You, R. Liu and J. Zhu, Readily recyclable, high-performance thermosetting materials based on a lignin-derived spiro diacetal trigger, J. Mater. Chem. A, 2019, 7(3), 1233–1243, 10.1039/c8ta07140c.
- A. Moreno, M. Morsali and M. H. Sipponen, Catalyst-Free Synthesis of Lignin Vitrimers with Tunable Mechanical Properties: Circular Polymers and Recoverable Adhesives, ACS Appl. Mater. Interfaces, 2021, 13(48), 57952–57961, DOI:10.1021/acsami.1c17412.
- J. Wu, X. Yu, H. Zhang, J. Guo, J. Hu and M.-H. Li, Fully Biobased Vitrimers from Glycyrrhizic Acid and Soybean Oil for Self-Healing, Shape Memory, Weldable, and Recyclable Materials, ACS Sustainable Chem. Eng., 2020, 8(16), 6479–6487, DOI:10.1021/acssuschemeng.0c01047.
- L. Jiang, C.-G. Wang, P. L. Chee, C. Qu, A. Z. Fok, F. H. Yong, Z. L. Ong and D. Kai, Strategies for lignin depolymerization and reconstruction towards functional polymers, Sustainable Energy Fuels, 2023, 7(13), 2953–2973, 10.1039/d3se00173c.
- W. Deng, H. Zhang, X. Wu, R. Li, Q. Zhang and Y. Wang, Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles, Green Chem., 2015, 17(11), 5009–5018, 10.1039/c5gc01473e.
- C. Weng, X. Peng and Y. Han, Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis, Biotechnol. Biofuels Bioprod., 2021, 14(1), 84, DOI:10.1186/s13068-021-01934-w.
- H. Geng, Y. Wang, Q. Yu, S. Gu, Y. Zhou, W. Xu, X. Zhang and D. Ye, Vanillin-Based Polyschiff Vitrimers: Reprocessability and Chemical Recyclability, ACS Sustainable Chem. Eng., 2018, 6(11), 15463–15470, DOI:10.1021/acssuschemeng.8b03925.
- Q. Yu, X. Peng, Y. Wang, H. Geng, A. Xu, X. Zhang, W. Xu and D. Ye, Vanillin-based degradable epoxy vitrimers: Reprocessability and mechanical properties study, Eur. Polym. J., 2019, 117, 55–63, DOI:10.1016/j.eurpolymj.2019.04.053.
- J. Liu and K. V. Bernaerts, Preparation of lignin-based imine vitrimers and their potential application as repairable, self-cleaning, removable and degradable coatings, J. Mater. Chem. A, 2024, 12, 2959–2973, 10.1039/d3ta06338k.
- L. Yuan, W. Zhou, Y. Shen and Z. Li, Chemically recyclable polyurethanes based on bio-renewable γ-butyrolactone: From thermoplastics to elastomers, Polym. Degrad. Stab., 2022, 204, 110116, DOI:10.1016/j.polymdegradstab.2022.110116.
- P. S. Choong, N. X. Chong, E. K. Wai Tam, A. M. Seayad, J. Seayad and S. Jana, Biobased Nonisocyanate Polyurethanes as Recyclable and Intrinsic Self-Healing Coating with Triple Healing Sites, ACS Macro Lett., 2021, 10(5), 635–641, DOI:10.1021/acsmacrolett.1c00163.
- Z. Wei, Z. Liang, Z. Feng, B. Xue, C. Xiong, C. Duan and Y. Ni, New Kind of Lignin/Polyhydroxyurethane Composite: Green Synthesis, Smart Properties, Promising Applications, and Good Reprocessability and Recyclability, ACS Appl. Mater. Interfaces, 2021, 13(24), 28938–28948, DOI:10.1021/acsami.1c06822.
- Y. Yang and M. W. Urban, Self-healing of glucose-modified polyurethane networks facilitated by damage-induced primary amines, Polym. Chem., 2017, 8(1), 303–309, 10.1039/c6py01221c.
- X. Meng, S. Zhang, B. Scheidemantle, Y.-Y. Wang, Y. Pu, C. E. Wyman, C. M. Cai and A. J. Ragauskas, Preparation and characterization of aminated co-solvent enhanced lignocellulosic fractionation lignin as a renewable building block for the synthesis of non-isocyanate polyurethanes, Ind. Crops Prod., 2022, 178, 114579, DOI:10.1016/j.indcrop.2022.114579.
- X. Yang, S. Wang, X. Liu, Z. Huang, X. Huang, X. Xu, H. Liu, D. Wang and S. Shang, Preparation of non-isocyanate polyurethanes from epoxy soybean oil: dual dynamic networks to realize self-healing and reprocessing under mild conditions, Green Chem., 2021, 23(17), 6349–6355, 10.1039/d1gc01936h.
- W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. M. Winne and F. E. Du Prez, Vinylogous Urethane Vitrimers, Adv. Funct. Mater., 2015, 25(16), 2451–2457, DOI:10.1002/adfm.201404553.
- P. Haida, S. Chirachanchai and V. Abetz, Starch-Reinforced Vinylogous Urethane Vitrimer Composites: An Approach to Biobased, Reprocessable, and Biodegradable Materials, ACS Sustainable Chem. Eng., 2023, 11(22), 8350–8361, DOI:10.1021/acssuschemeng.3c01340.
- P. Haida, G. Signorato and V. Abetz, Blended vinylogous urethane/urea vitrimers derived from aromatic alcohols, Polym. Chem., 2022, 13(7), 946–958, 10.1039/d1py01237a.
- L. Sougrati, A. Duval and L. Avérous, From Lignins to Renewable Aromatic Vitrimers based on Vinylogous Urethane, ChemSusChem, 2023, 16, 23, DOI:10.1002/cssc.202300792.
- J. Zheng, Z. M. Png, S. H. Ng, G. X. Tham, E. Ye, S. S. Goh, X. J. Loh and Z. Li, Vitrimers: Current research trends and their emerging applications, Mater. Today, 2021, 51, 586–625, DOI:10.1016/j.mattod.2021.07.003.
- N. J. Van Zee and R. Nicolaÿ, Vitrimer Chemistry and Applications, Macromol. Eng., 2022, 1–38 Search PubMed.
- S. Gao, Y. Liu, S. Feng and Z. Lu, Reprocessable and degradable thermoset with high Tg cross-linked via Si–O–Ph bonds, J. Mater. Chem., 2019, 7(29), 17498–17504, 10.1039/c9ta04951g.
| This journal is © The Royal Society of Chemistry 2024 |