Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A rechargeable self-healing safety fuel gel†
Santu
Bera
and
Debasish
Haldar
*
Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur 741252, West Bengal, India. E-mail: deba_h76@yahoo.com; deba_h76@iiserkol.ac.in; Fax: +91 3325873020; Tel: +91 3325873119
First published on 11th November 2015
Abstract
The stimuli responsive assembly of small molecules that can modulate the physical properties of a supramolecular system may have potential as futuristic materials. The dimethyl dipicolinate in alcohol, when combined with 3 equiv. of potassium hydroxide and water, aggregates to a strong self-healing transparent gel. The instant gelation is highly selective towards potassium hydroxide and does not need any heating–cooling or sonication. The gelation is also selective for solvent systems such as 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol–water or ethanol–water. The rheological stepstrain measurements under varying strain at room temperature show that the gel is thixotropic in nature. The gel can be shaped like any free-standing objects due to its high mechanical strength. The gel exhibits remarkable self-healing properties when damaged and diffusion of methyl orange through the gel suggests the dynamic nature of the gel. We found that the non-explosive gel can be packaged in a polyethylene tube and used as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it rechargeable up to four times by the addition of (9
1 methanol–water or ethanol–water. The rheological stepstrain measurements under varying strain at room temperature show that the gel is thixotropic in nature. The gel can be shaped like any free-standing objects due to its high mechanical strength. The gel exhibits remarkable self-healing properties when damaged and diffusion of methyl orange through the gel suggests the dynamic nature of the gel. We found that the non-explosive gel can be packaged in a polyethylene tube and used as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it rechargeable up to four times by the addition of (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) methanol–water.
1) methanol–water.
1. Introduction
Molecular recognition and self-assembly processes are key for developing new functional materials such as supramolecular gels.1–9 Generally supramolecular gels are entangled networks of fibers composed of self-assembled building blocks that can entrap relatively large amounts of solvents.10–12 Due to the reversibility of the formation through noncovalent interactions and their degradability, homogeneity, and tunability13 the supramolecular gels have excellent responsiveness to different stimuli, such as pH, light, enzyme, ions, temperature, ultrasound and mechanical strength.14 The supramolecular gels have potential applications including sensing of biomolecules and ions, light-harvesting systems,15–19 catalysis,20 optoelectronic devices,21 and as media for cell culture, tissue engineering and targeted drug delivery.22–29 Many organic motifs such as steroids,30 peptides,31–34 ureas and amides,35–40 sugars,41 dendrimers,42,43 and π-conjugated molecules44,45 have been designed and developed as gelatos. But, most of these organic gelator molecules need to be synthesized by tedious procedures. Exploration of simple and effective gelator molecules with novel functions is highly challenging.46–48Historically alcohols are known as fuel. Methanol and ethanol can be obtained from fossil fuels and biomass or industrially from CO2 and water. They have high octane rating and advantages over fuels such as petrol and diesel. As an alternative of hydrogen, methanol has been proposed as a future green fuel. However, their liquid nature and relatively high volatility make them inconvenient to use for different purposes. There are also issues with spilling and safety. Hence, converting liquid alcohols into gel will be highly advantageous for easy handling, delivery and safety.
Herein we report one stimuli responsive simple gelator that can congeal an alcohol–water mixture to give a strong, self-supporting, and transparent gel with magnificent self-healing properties. The gelation is highly selective for potassium hydroxide. Interestingly, the assembly of gelator molecules can effectively trap methanol or ethanol in the presence of water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The gel exhibits high stability, thixotropic behaviour and self-healing properties. We also demonstrate novel applications of this gel as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it reusable up to four times by the addition of (9
1). The gel exhibits high stability, thixotropic behaviour and self-healing properties. We also demonstrate novel applications of this gel as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it reusable up to four times by the addition of (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) methanol/water. The eco-friendly gel can be coloured with organic dyes and can be packed in any container.
1) methanol/water. The eco-friendly gel can be coloured with organic dyes and can be packed in any container.
2. Results and discussion
For instant gelation, we chose a non-covalent approach. The greener part is that the gelation does not need any heating–cooling cycle or sonication. The gel consists of four components: methanol, water, dimethyl dipicolinate and potassium hydroxide. Dipicolinic acid is a naturally occurring chemical (composes 5% to 15% of bacterial spores) and responsible for the heat resistance of bacterial endospore, and is cheap to purchase. On mixing with potassium hydroxide in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol–water, an instant transparent gel appeared. The most exciting fact is that the gelation is very selective for KOH only as other bases like NaOH, LiOH, K2CO3, Na2CO3, and ET3N fail to form any such type of gel under similar conditions. This suggests that both potassium and hydroxide ions have a significant role in the self-assembly process to form a soft gel phase material. In order to determine the optimal molar composition of the three variables (water, methanol and KOH), we first carried out an extensive three-variables screening defined by the ratio of water and methanol and the number of equivalents of KOH.
1 methanol–water, an instant transparent gel appeared. The most exciting fact is that the gelation is very selective for KOH only as other bases like NaOH, LiOH, K2CO3, Na2CO3, and ET3N fail to form any such type of gel under similar conditions. This suggests that both potassium and hydroxide ions have a significant role in the self-assembly process to form a soft gel phase material. In order to determine the optimal molar composition of the three variables (water, methanol and KOH), we first carried out an extensive three-variables screening defined by the ratio of water and methanol and the number of equivalents of KOH.
Initially, 6 mg (1 equiv.) of dimethyl dipicolinate (compound 1) and an excess of KOH (5 equiv.) were taken and the gelation ability was studied in 1 mL solvent by changing the ratio of water and methanol in a stepwise fashion. In workup methanol without any percentage of water, the compound fails to form a gel. Addition of water causes the formation of a soft gel material. When the ratio of water and methanol is 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5, the compound forms a partial gel and half of the solvent remains free. The gelation ability increases with increasing ratio of water in methanol. The best gel is obtained when the ratio of water and methanol is 1
9.5, the compound forms a partial gel and half of the solvent remains free. The gelation ability increases with increasing ratio of water in methanol. The best gel is obtained when the ratio of water and methanol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. But after this ratio, further increase of water causes inhibition of gel formation (Fig. 1). When the ratio reaches 1.5
9. But after this ratio, further increase of water causes inhibition of gel formation (Fig. 1). When the ratio reaches 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5, the whole of the system remains in a liquid phase without the formation of gel. The transparency of the gel material also increases with increase in the percentage of water in methanol (ESI Fig. 1†).
8.5, the whole of the system remains in a liquid phase without the formation of gel. The transparency of the gel material also increases with increase in the percentage of water in methanol (ESI Fig. 1†).
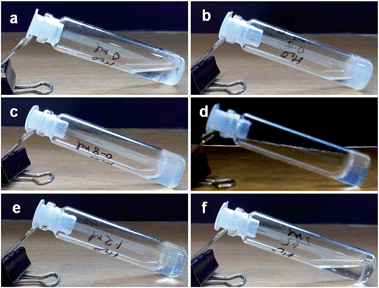 | ||
Fig. 1 Gelation study of compound 1 (6 mg, 1 equiv.) and KOH (5 equiv.) with various ratios of water and methanol (a) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, (b) 0.5 10, (b) 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5, (c) 0.8 9.5, (c) 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.2, (d) 1 9.2, (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, (e) 1.2 9, (e) 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.8, and (f) 1.5 8.8, and (f) 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5. 8.5. | ||
The gelation behaviour in the presence of other bases was also studied. Other bases including NaOH, LiOH, K2CO3, Na2CO3, and ET3N were also examined to address the question of whether any of them is able to form or induce the gelation. However, the compound 1 fails to form any kind of gel in the presence of the above-mentioned bases. This indicates that the gelation is extremely selective towards KOH. Moreover, the gelation of compound 1 was studied separately in K2CO3 and LiOH to examine whether the presence of K+ or OH− alone was sufficient for gel formation. But the compound fails to form a gel in K2CO3 and LiOH separately. However, when these two bases were used together for a gelation study, the compound formed a gel (Fig. 2). This suggests that K2CO3 and LiOH work as a source of K+ and OH−, respectively, and the gelation is highly selective towards KOH. Hence K+ and OH− ions have a significant effect in the self-assembly process of compound 1 in the gel state.
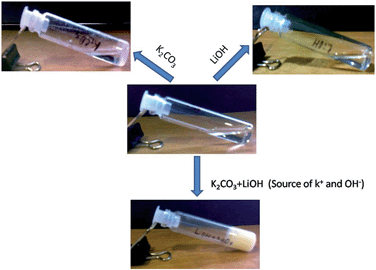 | ||
Fig. 2 Gelation study of compound 1 (2% w/v) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, ratio of water and methanol in the presence of K+ and OH− ions. 9, ratio of water and methanol in the presence of K+ and OH− ions. | ||
The composition and stoichiometry for gel formation were further studied by varying the equiv. of KOH. The minimum equivalent of KOH required for the gelation was screened by taking 20 mg of compound 1 in 1 mL of solvent (water and methanol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and by increasing the equivalent of KOH from 0 to 5 equivalents to that of compound 1 (ESI Fig. 2†). It was found that a minimum of 3.5 equivalent of KOH was required for the gelation. The minimum gelation concentration (MGC) at room temperature was determined by using 3 equiv. of KOH and 1
9) and by increasing the equivalent of KOH from 0 to 5 equivalents to that of compound 1 (ESI Fig. 2†). It was found that a minimum of 3.5 equivalent of KOH was required for the gelation. The minimum gelation concentration (MGC) at room temperature was determined by using 3 equiv. of KOH and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 water/methanol as the solvent. In a methanolic solution of compound 1, the addition of aqueous KOH (3 equiv.), where the ultimate ratio of water/methanol is 1
9 water/methanol as the solvent. In a methanolic solution of compound 1, the addition of aqueous KOH (3 equiv.), where the ultimate ratio of water/methanol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, instantly transforms it into a gel material without any external stimuli like heat or sonication. The minimum gelation concentration (MGC) was 0.5% w/v. The obtained gel material is not thermo-reversible and it is very stable at a comparatively high temperature.
9, instantly transforms it into a gel material without any external stimuli like heat or sonication. The minimum gelation concentration (MGC) was 0.5% w/v. The obtained gel material is not thermo-reversible and it is very stable at a comparatively high temperature.
A wide variety of alcoholic solvents were used to make the gel of compound 1 in the presence of KOH and water. However, the compound formed a gel only in methanol and ethanol. In other alcohols like 1-propanol, 2-propanol, and 1-butanol, the compound 1 fails to form a gel under the same conditions (Fig. 3). The gelation has been confirmed by the inverted vial method.49,50 The opaque or transparent gels are stable for 2–3 months at room temperature. However, picolinic acid or monomethyl picolinate has failed to form a KOH responsive gel under the same conditions.
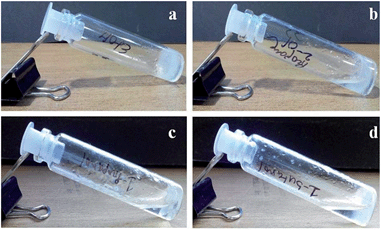 | ||
Fig. 3 Gelation study of compound 1 (2% w/v) and KOH (3.5 equiv.) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of water and (a) ethanol, (b) 2-propanaol, (c) 1-propanol, (d) 1-butanol. 9 ratio of water and (a) ethanol, (b) 2-propanaol, (c) 1-propanol, (d) 1-butanol. | ||
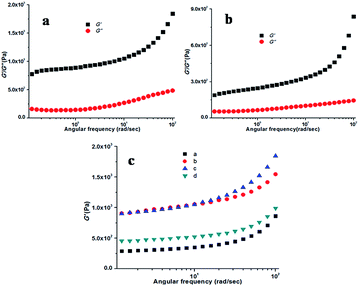
The visco-elastic properties of the gels were derived primarily from the tertiary structure by different rheological studies.51 Rheology is the study of flow that provides information about the type of network (tertiary structure) responsible for the gelation. All the experiments were performed using a freshly prepared KOH responsive gel. By measuring the response of the soft material to an applied oscillatory stress, several variables can be resolute. The storage modulus (G′, a measure of the elastic response of the material) and loss modulus (G′′, a measure of the viscous response) were measured as a function of shear stress and strain at 20 °C and a frequency of 10 rad s−1. For both the KOH responsive methanol and ethanol gel of compound 1, the storage modulus (G′) was found to be approximately an order of magnitude larger than the loss modulus (G′′), indicative of an elastic material and this is the comportment expected for a true gel phase (Fig. 4). G′ and G′′ remain almost invariant with an increase in the applied stress. Throughout the experiment G′ and G′′ do not cross each other, suggesting the presence of a stable and rigid gel phase material.
Thixotropic behaviour of gel was established by rheological measurements. A thixotropic gel is one which has the ability to disintegrate to solution or a quasi-liquid state under some external stimuli and can regain its original shape after the removal of the applied stimuli. This process can be continued for several numbers of cycles via breaking and reconstructing the gels. In order to establish the thixotropic properties of the present gels, we have performed rheological step strain measurements52,53 under varying strain at 20 °C. At first gels were subjected to a constant strain of 0.1%. At this strain G′ is higher than G′′ (step 1, Fig. 5). Then the strain was increased from 0.1% to 30% and was kept for few minutes at 30% strain to break gels completely which was confirmed by complete inversion of G′ and G′′ (step 2, Fig. 5). Then the strain was decreased from 30% to 0.1% again and kept for few minutes at 0.1% to observe the gel restoration kinetics (step 3, Fig. 5). After releasing the high strain the sol state again transformed into a semi-solid state and quickly regained its mechanical strength and we found almost 80% recovery at the low strain of 0.1%. This was continued for several cycles.
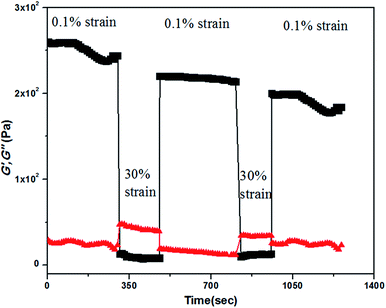 | ||
Fig. 5 The step strain experimental data obtained from the KOH responsive gel of compound 1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 water 9 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH. MeOH. | ||
The morphological features of these KOH responsive gels were investigated by the field emission scanning electron microscopy studies (FE-SEM).54 For FE-SEM experiments, to avoid any possible artefacts we executed experiments with a lowest KOH concentration containing diluted gel. The FE-SEM images of xerogel exhibit tape-like nanofibrillar network structures. The nanofibrills are polydisperse in nature and highly entangled. Interestingly, the fibres are twisted into helical structures (Fig. 6). Generally helical gel fibres are observed for chiral gelators, since chirality is often translated from the molecular level to the supramolecular level. However, compound 1 is not chiral and both left and right handed helical architectures are observed throughout the xerogel in every sample examined. In the absence of molecular chirality, the twisting may be responsible for the left and right handed helical architectures of the resultant fibers. The FE-SEM images of the xerogel obtained from different organic solvents show that the network morphology of peptide 1 does not vary from solvent to solvent.
 | ||
Fig. 6 The FE-SEM images of the xerogel showing a twisted fibrillar network structure of KOH responsive compound 1 gel in (a) and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 water 9 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH and (c) and (d) 1 MeOH and (c) and (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 water 9 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH. EtOH. | ||
Titration of compound 1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 D2O/MeOD with increasing amount of KOH monitored by 1H NMR spectroscopy provided evidence for the KOH compound 1 interaction. The aromatic proton signals of compound 1 exhibited further splitting without any change of integration and shifted gradually towards downfield with a gradual increase in KOH concentration, thus supporting the self-assembly through KOH compound 1 interactions (ESI Fig. 3†). A similar result was obtained by titration with LiOH (ESI Fig. 4†). Only, after the addition of 2.5 equivalents of LiOH, the aromatic proton signals of compound 1 return back to its original shape. The ester proton signal of compound 1 also shifted downfield with the addition of KOH or LiOH, without any change of integration suggesting that there is no ester hydrolysis under this condition. This is further supported by the existence of a FTIR signal at 1653 cm−1 responsible for ester carbonyls in both solution and xerogel (ESI Fig. 5†). Mass spectrometry of the xerogel has confirmed the absence of hydrolyzed products. Moreover, to gain better insight into the morphological evolution by KOH responsive compound 1 gel formation, X-ray diffraction experiments have been performed on the xerogel.55,56 The powder X-ray diffraction (PXRD) pattern of the xerogel clearly shows that the material is crystalline in the xerogel and sharp reflections appeared in the 5–40° 2θ range. Sharp peaks appear at 2θ = 7.08 and 12.13 with long d spacing values of 12.47 Å and 7.29 Å, respectively (ESI Fig. 6†). These two peaks indicate the presence of a sheet-like packing arrangement.57–59 A comparison of the spectrum obtained from the powder X-ray diffraction data of the xerogel and the powder pattern from X-ray crystallography of a single crystal of compound 1 and a single crystal of KOH clearly shows the significant differences and existence of KOH compound 1 interactions. Scanning electron microscopy images of the xerogels show twisted tape-like fibers. Energy-dispersive X-ray spectroscopy (EDS) experiments have been carried out for metal content determination of the xerogel. EDS confirms the potassium content (ESI Fig. 7†). Considering the above mentioned experiments a tentative model has been developed (ESI Fig. 8†).
9 D2O/MeOD with increasing amount of KOH monitored by 1H NMR spectroscopy provided evidence for the KOH compound 1 interaction. The aromatic proton signals of compound 1 exhibited further splitting without any change of integration and shifted gradually towards downfield with a gradual increase in KOH concentration, thus supporting the self-assembly through KOH compound 1 interactions (ESI Fig. 3†). A similar result was obtained by titration with LiOH (ESI Fig. 4†). Only, after the addition of 2.5 equivalents of LiOH, the aromatic proton signals of compound 1 return back to its original shape. The ester proton signal of compound 1 also shifted downfield with the addition of KOH or LiOH, without any change of integration suggesting that there is no ester hydrolysis under this condition. This is further supported by the existence of a FTIR signal at 1653 cm−1 responsible for ester carbonyls in both solution and xerogel (ESI Fig. 5†). Mass spectrometry of the xerogel has confirmed the absence of hydrolyzed products. Moreover, to gain better insight into the morphological evolution by KOH responsive compound 1 gel formation, X-ray diffraction experiments have been performed on the xerogel.55,56 The powder X-ray diffraction (PXRD) pattern of the xerogel clearly shows that the material is crystalline in the xerogel and sharp reflections appeared in the 5–40° 2θ range. Sharp peaks appear at 2θ = 7.08 and 12.13 with long d spacing values of 12.47 Å and 7.29 Å, respectively (ESI Fig. 6†). These two peaks indicate the presence of a sheet-like packing arrangement.57–59 A comparison of the spectrum obtained from the powder X-ray diffraction data of the xerogel and the powder pattern from X-ray crystallography of a single crystal of compound 1 and a single crystal of KOH clearly shows the significant differences and existence of KOH compound 1 interactions. Scanning electron microscopy images of the xerogels show twisted tape-like fibers. Energy-dispersive X-ray spectroscopy (EDS) experiments have been carried out for metal content determination of the xerogel. EDS confirms the potassium content (ESI Fig. 7†). Considering the above mentioned experiments a tentative model has been developed (ESI Fig. 8†).
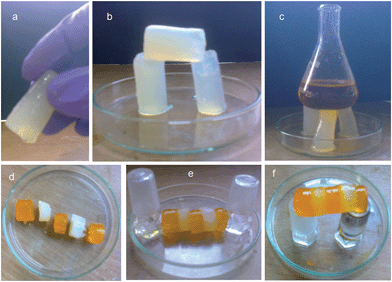
Interestingly, the KOH responsive gels formed by compound 1 from water/methanol at a low concentration (1 wt%) are so stable that they can be shaped into any self-supporting geometrical shape using appropriate molds.60 The gel could be handled with a tweezer or suspended in air just by holding one side (Fig. 7a). Also a big gel block can be sliced into various small pieces with a razor (Fig. 7b).61 The KOH responsive gels formed by compound 1 from water/methanol at a low concentration (1 wt%) are sufficiently strong to tolerate the weight of several grams (Fig. 7c). More interestingly, these gels showed remarkable self-healing properties. When a block of gel was cut into two pieces and then joined together, the pieces merged into a continuous block.61 Also, several small blocks of gels could be joined together to form a stable self-supporting bar. These fused bars could be taken in hand or suspended in air just by holding one side. The fusion of a methyl orange-doped gel with an undoped gel established the diffusion of methyl orange to the undoped piece, thus suggesting the dynamic exchange of dissolved molecules across the fusion interface (Fig. 7d–f). This self-healing process shows that the gel is living and dynamic in nature.62,63 There is a dynamic equilibrium between continuous formation of new fibers and dissociation of old fibers. This movement would allow the growth of new fibers across the fusion interface and thus helps the repairing process.
The selectivity of compound 1 for forming a gel only in methanol or ethanol makes it an interesting candidate in separation of various miscible organic solvents.64 If we have a mixture of ethyl acetate and methanol, we can separate the methanol by forming a gel. By the addition of a little amount of compound 1 and aqueous KOH to this ethyl acetate–methanol mixture, the compound will form a gel only with methanol and precipitate out. This gel can be removed easily from ethyl acetate by decantation or simple filtration (ESI Fig. 8†). Also the methanol can be collected by distillation of the gel. To the best of our knowledge this is the greenest way to separate miscible organic solvents.
To study the role of ester functional groups in compound 1 for gelation we have studied different analogues like mono acid mono ester and diacid of picolinic acid. In this regard we have studied compound 2 where monomethyl picolinate esters are separated by a spacer hexamethylenediamine. This compound does not form KOH responsive gels in a water/methanol mixture of any composition and even at a very high concentration. But, from X-ray crystallography, it is evident that the asymmetric unit contains two molecules of compound 2 and two molecules of water (ESI Fig. 10†). The hexamethylenediamine adopts all anti-conformation. There are two intramolecular five member hydrogen bonds between pyridine N and hexamethylenediamine NH (N2–H2⋯˙N1). The water molecule acts as a bridge between two molecules of compound 2 by two intermolecular hydrogen bonding interactions (O1S–H40⋯˙O2) and thereby forms a sheet-like structure in a higher order assembly (ESI Fig. 11†). We have correlated the crystal structure of compounds 1 and 2. Compound 1 also shows a malty layer sheet-like structure in a higher order packing in the crystal (ESI Fig. 12†). As the ester oxygens involve in hydrogen bonding with water molecules, the compound 2 does not interact with KOH in a similar way as compound 1. The compounds containing monomethyl picolinate and ethylenediamine65 or propylenediamine65 also failed to form KOH responsive gels in a water/methanol mixture.
Alcohols have been used as fuels for a long time and have high octane rating and some advantages over petrol and diesel. However, there are some limitations of using alcohols due to liquidity, high volatility, spilling and safety. Hence, converting the liquid alcohols into gels will be highly advantageous for easy handling, delivery and safety. On burning, the KOH responsive compound 1 methanol or ethanol gel does not produce any shoot, smoke, obnoxious odour and ash. The gelling agents are non-explosive, non-carcinogenic and non-corrosive. Due to its high mechanical strength, the gel can be shaped like any free-standing objects and can be packed in any container even in plastic jars, tubes and bottles. The gel exhibits remarkable self-healing properties. This dynamic nature of the gel made it practically useful as fuel-like paraffin. We found that the non explosive gel can be used as a safe fuel for indoor and outdoor heating without any special stove or burner (Fig. S13, ESI†). The KOH responsive compound 1 in ethanol has 1300 kJ mol−1 heat of combustion compared to 726 kJ mol−1 KOH for responsive compound 1 in methanol. Moreover, different organic colours (like methyl orange or rhodamine 6G) can be added to make the fuel gel colourful and more attractive. The gelator retains strong memory even after burning which makes it reusable up to four times by the addition of (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) methanol–water (Fig. 8). The compound 1 contained an ester segment, which was hydrolyzed by KOH in the burning process at high temperature and the gelation efficiency decreased with increasing burning cycles (Fig. S14, ESI†). The problem with other available normal heating–cooling alcoholic gels used as fuel gels is that with the start of burning the whole gel is converted to liquid and burns completely within a short time. As the KOH responsive compound 1 methanol or ethanol gel is not thermo-reversible and stable at high temperature, it remains in a gel phase at the time of burning and takes a long time to finish. Therefore the KOH responsive compound 1 methanol or ethanol gel is more user friendly and appealing to the consumers.
1) methanol–water (Fig. 8). The compound 1 contained an ester segment, which was hydrolyzed by KOH in the burning process at high temperature and the gelation efficiency decreased with increasing burning cycles (Fig. S14, ESI†). The problem with other available normal heating–cooling alcoholic gels used as fuel gels is that with the start of burning the whole gel is converted to liquid and burns completely within a short time. As the KOH responsive compound 1 methanol or ethanol gel is not thermo-reversible and stable at high temperature, it remains in a gel phase at the time of burning and takes a long time to finish. Therefore the KOH responsive compound 1 methanol or ethanol gel is more user friendly and appealing to the consumers.
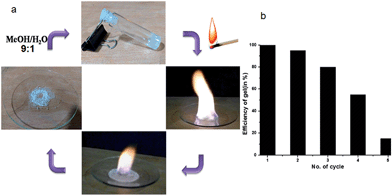 | ||
Fig. 8 (a) The burning methanol gel and recharge by 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol–water. (b) Efficiency of gel after various cycles. 1 methanol–water. (b) Efficiency of gel after various cycles. | ||
3. Experimental
General
All chemicals were purchased from Sigma chemicals.Synthesis
The compound 1 was synthesized from picolinic acid by conventional methods. The compound 2 was synthesized from monomethyl picolinate and hexamethylenediamine by a solution-phase methodology. The products were purified by column chromatography using a silica (100–200 mesh size) gel as a stationary phase and an n-hexane–ethyl acetate mixture as an eluent. The compounds were fully characterized by 500 MHz and 400 MHz 1H NMR spectroscopy, 125 MHz 13C NMR spectroscopy, FTIR spectroscopy and mass spectrometry. The compounds 1 and 2 were characterized by X-ray crystallography.NMR experiments
All NMR studies were carried out on a Brüker AVANCE 500 MHz spectrometer. Compound concentrations were in the range of 1–10 mm in CD3OD, MeOD and D2O.FTIR spectroscopy
All reported solid-state FTIR spectra were obtained with a Perkin Elmer Spectrum RX1 spectrophotometer using the KBr disk technique and in MeOH solution.Mass spectrometry
Mass spectra were recorded on a Q-Tof Micro YA263 high-resolution (Waters Corporation) mass spectrometer by positive-mode electrospray ionization.Gelation
The compound 1 (dimethyl dipicolinate) (6 mg, 1 equiv.) and an excess of KOH (5 equiv.) were taken and the gelation ability was studied in 1 mL solvent by changing the ratio of water and methanol in a stepwise fashion at room temperature. An instant homogeneous gel appeared when the ratio of water and methanol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.
9.
Rheology
The viscoelastic properties of sonication induced gels were measured with a commercial rheometer (AR-G2, TA Instruments, New Castle, USA).Single crystal X-ray diffraction study
Intensity data were collected with MoKα radiation using a Bruker APEX-2 CCD diffractometer. Data were processed using the Bruker SAINT package and the structure solution and refinement procedures were performed using SHELX97. The non-hydrogen atoms were refined with anisotropic thermal parameters. The data have been deposited at the Cambridge Crystallographic Data Centre with reference CCDC 918260.Field emission scanning electron microscopy
Morphologies of the reported xerogel were investigated using field emission-scanning electron microscopy (FE-SEM). A small amount of sample was placed on a clean glass slide and then dried by slow evaporation. The material was then allowed to dry under vacuum at 30 °C for two days. The materials were gold-coated, and the micrographs were taken in an FE-SEM apparatus (Jeol Scanning Microscope-JSM-6700F).4. Conclusions
In conclusion, we have developed a stimuli responsive organogelation system at room temperature based on dimethyl picolinate. The gelation is highly selective for potassium hydroxide. Interestingly, the assembly of gelator molecules can effectively trap methanol or ethanol in the presence of water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The gel exhibits high stability, thixotropic behaviour and self-healing properties. The non-explosive gel can be used as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it rechargeable up to four times by the addition of (9
1). The gel exhibits high stability, thixotropic behaviour and self-healing properties. The non-explosive gel can be used as a safe fuel for indoor and outdoor heating without any special stove or burner. The gelator retains strong memory even after burning which makes it rechargeable up to four times by the addition of (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) methanol–water. The eco-friendly gel can be shaped into any self-supporting geometrical shape, coloured with organic dyes and can be packed in any container which is appealing to the consumers.
1) methanol–water. The eco-friendly gel can be shaped into any self-supporting geometrical shape, coloured with organic dyes and can be packed in any container which is appealing to the consumers.
Acknowledgements
We acknowledge the CSIR, India, for financial assistance (Project No. 01/2507/11-EMR-II). S. Bera thanks the UGC, India for research fellowship.Notes and references
- R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed.
- J. Raeburn and D. J. Adams, Chem. Commun., 2014, 51, 5170–5180 RSC.
- D. J. Cornwell and D. K. Smith, Mater. Horiz., 2015, 2, 279–293 RSC.
- G. O. Lloyd and J. W. Steed, Nat. Chem., 2009, 1, 437–442 CrossRef CAS PubMed.
- L. A. Estroff and A. D. Hamilton, Chem. Soc. Rev., 2004, 104, 1201–1216 CrossRef CAS PubMed.
- G. Yu, X. Yan, C. Han and F. Huang, Chem. Soc. Rev., 2013, 42, 6697–6722 RSC.
- L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC.
- J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- Y. Hisamatsu, S. Banerjee, M. B. Avinash, T. Govindaraju and C. Schmuck, Angew. Chem., Int. Ed., 2013, 52, 12550–12554 CrossRef CAS PubMed.
- K. L. Caran, D.-C. Lee and R. G. Weiss, Molecular Gels and Their Fibrillar Networks, in Soft Fibrillar Materials: Fabrication and Applications, ed. X. Y. Liu and J.-L. Li, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 Search PubMed.
- R. G. Weiss and P. Terech, Molecular Gels: Materials With Self-assembled Fibrillar Networks, Springer, Dordrecht, The Netherlands, 2006 Search PubMed.
- S. R. Raghavan and J. F. Douglas, Soft Matter, 2012, 8, 8539–8546 RSC.
- A. S. Weingarten, R. V. Kazantsev, L. C. Palmer, M. McClendon, A. R. Koltonow, A. P. S. Samuel, D. J. Kiebala, M. R. Wasielewski and S. I. Stupp, Nat. Chem., 2014, 6, 964–970 CrossRef CAS PubMed.
- M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086–7098 RSC.
- G. M. Peters, L. P. Skala, T. N. Plank, H. Oh, G. N. M. Reddy, A. Marsh, S. P. Brown, S. R. Raghavan and J. T. Davis, J. Am. Chem. Soc., 2015, 137, 5819–5827 CrossRef CAS PubMed.
- M. Ikeda, T. Tanida, T. Yoshii, K. Kurotani, S. Onogi, K. Urayama and I. Hamachi, Nat. Chem., 2014, 6, 511–518 CrossRef CAS PubMed.
- M. Guo, L. M. Pitet, H. M. Wyss, M. Vos, P. Y. W. Dankers and E. W. J. Meijer, J. Am. Chem. Soc., 2014, 136, 6969–6977 CrossRef CAS PubMed.
- W. Guo, C.-H. Lu, X.-J. Qi, R. Orbach, M. Fadeev, H.-H. Yang and I. Willner, Angew. Chem., Int. Ed., 2014, 53, 10134–10138 CrossRef CAS PubMed.
- T. Yoshii, M. Ikeda and I. Hamachi, Angew. Chem., Int. Ed., 2014, 53, 7264–7267 CrossRef CAS PubMed.
- S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766–1776 CrossRef CAS PubMed.
- D. D. Diaz, D. Kuehbeck and R. J. Koopmans, Chem. Soc. Rev., 2011, 40, 427–448 RSC.
- T. Yoshii, S. Onogi, H. Shigemitsu and I. Hamachi, J. Am. Chem. Soc., 2015, 137, 3360–3365 CrossRef CAS PubMed.
- G.-F. Liu, D. Zhang and C.-L. Feng, Angew. Chem., Int. Ed., 2014, 53, 7789–7793 CrossRef CAS PubMed.
- Y. Kuang, J. Shi, J. Li, K. A. Alberti, Q. Xu and B. Xu, Angew. Chem., Int. Ed., 2014, 53, 8104–8107 CrossRef CAS PubMed.
- L. E. Buerkle, H. A. von Recum and S. J. Rowan, Chem. Sci., 2012, 3, 564–572 RSC.
- M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670–1673 CrossRef CAS PubMed.
- H. K. Lau and K. L. Kiick, Biomacromolecules, 2015, 16, 28–42 CrossRef CAS PubMed.
- K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellam and M. Marlow, Soft Matter, 2014, 10, 237–256 RSC.
- N. M. Sangeeth and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- H. Svobodova, V. Noponen, E. Kolehmainen and E. Sievanen, RSC Adv., 2012, 2, 4985–5007 RSC.
- Y. Li, B. Z. Li, Y. T. Fu, S. W. Lin and Y. G. Yang, Langmuir, 2013, 29, 9721–9726 CrossRef CAS PubMed.
- K. Isozaki, H. Takaya and T. Naota, Angew. Chem., Int. Ed., 2007, 46, 2855–2857 CrossRef CAS PubMed.
- C. Kang, L. Wang, Z. Bian, H. Guo, X. Ma, X. Qiu and L. Gao, Chem. Commun., 2014, 50, 13979–13982 RSC.
- L. E. R. O'Leary, J. A. Fallas, E. L. Bakota, M. K. Kang and J. D. Hartgerink, Nat. Chem., 2011, 3, 821–828 CrossRef PubMed.
- J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC.
- N. S. Vujicic, Z. Glasovac, N. Zweep, J. H. van Esch, M. Vinkovic, J. Popovic and M. Zinic, Chem.–Eur. J., 2013, 19, 8558–8572 CrossRef CAS PubMed.
- X. Wang and M. Liu, Chem.–Eur. J., 2014, 20, 10110–10116 CrossRef CAS PubMed.
- Z. Shen, T. Wang and M. Liu, Angew. Chem., Int. Ed., 2014, 53, 13424–13428 CrossRef CAS PubMed.
- S. J. James, A. Perrin, C. D. Jones, D. S. Yufit and J. W. Steed, Chem. Commun., 2014, 50, 12851–12854 RSC.
- L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42–47 CrossRef CAS PubMed.
- J. Cui, J. Zheng, W. Qiao and X. Wan, J. Colloid Interface Sci., 2008, 326, 267–274 CrossRef CAS PubMed.
- J. Liu, Y. Feng, Z. Liu, Z. Yan, Y. He, C. Liu and Q. Fan, Chem.–Asian J., 2013, 8, 572–581 CrossRef CAS PubMed.
- Z. Ding, R. Xing, X. Wang, J. Ding, L. Wang and Y. Han, Soft Matter, 2013, 9, 10404–10412 RSC.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed.
- C. Kang, Z. Bian, Y. He, F. Han, X. Qiu and L. Gao, Chem. Commun., 2011, 47, 10746–10748 RSC.
- A. A. Bredikhin, Z. A. Bredikhina, F. S. Akhatova and A. T. Gubaidullin, Chem. Commun., 2010, 46, 3523–3525 RSC.
- Y. Ohsedo, M. Miyamoto, M. Oono, K. Shikii and A. Tanaka, RSC Adv., 2013, 3, 3844–3847 RSC.
- P. W. J. M. Frederix, G. G. Scott, Y. M. Abul-Haija, D. Kalafatovic, C. G. Pappas, N. Javid, N. T. Hunt, R. V. Ulijn and T. Tuttle, Nat. Chem., 2015, 7, 30–37 CrossRef CAS PubMed.
- A. Pramanik, A. Paikar and D. Haldar, RSC Adv., 2015, 5, 53886–53892 RSC.
- A. Paikar, A. Pramanik and D. Haldar, RSC Adv., 2015, 5, 31845–31851 RSC.
- N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, J. Am. Chem. Soc., 2013, 135, 8989–8999 CrossRef CAS PubMed.
- N. T. Qazvini, S. Bolisetty, J. Adamcik and R. Mezzenga, Biomacromolecules, 2012, 13, 2136–2147 CrossRef CAS PubMed.
- S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 2356–2359 RSC.
- Z. Ma, P. Zhang, X. Yu, H. Lan, Y. Li, D. Xie, J. Lib and T. Yi, J. Mater. Chem. B, 2015, 3, 7366–7371 RSC.
- Q. Jin, J. Li, L. Zhang, S. Fanga and M. Liu, CrystEngComm, 2015, 17, 8058–8063 RSC.
- M. Dubey, A. Kumar, R. K. Gupta and D. S. Pandey, Chem. Commun., 2014, 50, 8144–8147 RSC.
- S. K. Samanta and S. Bhattacharya, Chem. Commun., 2013, 49, 1425–1427 RSC.
- B. O. Okesola and D. K. Smith, Chem. Commun., 2013, 49, 11164–11166 RSC.
- S. Basak, N. Nandi and A. Banerjee, Chem. Commun., 2014, 50, 6917–6919 RSC.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS PubMed.
- A. Vidyasagar, K. Handore and K. M. Sureshan, Angew. Chem., Int. Ed., 2011, 50, 8021–8024 CrossRef CAS PubMed.
- X. Yu, L. Chen, M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346–5371 RSC.
- Z. Xu, J. Peng, N. Yan, H. Yu, S. Zhang, K. Liu and Y. Fang, Soft Matter, 2013, 9, 1091–1099 RSC.
- S. Song, A. Song, L. Feng, G. Wei, S. Dong and J. Hao, ACS Appl. Mater. Interfaces, 2014, 6, 18319–18328 Search PubMed.
- S. Bera, S. Maity and D. Haldar, CrystEngComm, 2015, 17, 1569–1575 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compound 1, 1H NMR, 13C NMR, Fig. ESI S1–S14, and Fig. S1–S6. CCDC 918260. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ta08010j |
| This journal is © The Royal Society of Chemistry 2016 |