Metal-free 1,3-dipolar cycloaddition approach towards the regioselective synthesis of β-carboline and isoxazole based molecular hybrids†
Dharmender Singha,
Nisha Devia,
Vipin Kumara,
Chandi C. Malakarb,
Saloni Mehrac,
Ravindra K. Rawald,
B. S. Kaitha and
Virender Singh*a
aDepartment of Chemistry, Dr B. R. Ambedkar National Institute of Technology (NIT) Jalandhar, 144011, Punjab, India. E-mail: singhv@nitj.ac.in; Tel: +91 181 2690301 Tel: +91 181 2690320
bDepartment of Chemistry, National Institute of Technology (NIT) Manipur, Imphal 795004, Manipur, India
cAmity Institute of Applied Sciences, Amity University, Noida, 201313 U.P., India
dDepartment of Pharmaceutical Chemistry, Indo-Soviet Friendship College of Pharmacy, Moga 142001, Punjab, India
First published on 30th August 2016
Abstract
Nature has nourished β-carboline and isoxazole derivatives as privileged scaffolds and consequently they are ubiquitously found in alkaloids isolated from various sources. Moreover, several drug molecules based on them have been released in the market. Considering their immense impact, novel β-carboline–isoxazole-based molecular hybrids have been designed and a 1,3-dipolar cycloaddition strategy was devised to prepare the desired prototypes. A library of compounds with a wide range of diversity have been developed by employing β-carboline-containing dipolarophiles as well as dipoles. The current method represents a simple, efficient and easy-to-execute protocol towards the regioselective synthesis of β-carboline–isoxazole conjugates.
Introduction
The literature shows that the β-carboline nucleus is one of the most prominent heterocyclic frameworks in chemistry and constitutes more than one-third of the total alkaloids isolated from various natural sources, such as from terrestrial and aquatic plants, marine species, insects and mammals, including human beings (Fig. 1).1 β-Carboline-containing alkaloids exhibit a broad spectrum of pharmacological properties, such as sedative, anticonvulsant, anxiolytic, anti-tumour, anti-malarial, anti-leishmanial, anti-microbial and anti-HIV.2 Interestingly, the majority of these alkaloids display potent cytotoxic properties by multiple action mechanisms, such as intercalation into DNA strands, inhibiting cyclin-dependent kinases and topoisomerases and interacting with MK-2, kinesin-like protein Eg5 and PLK1, etc.3 More importantly, the β-carboline framework is also represented in commercial drugs, such as Tadalafil and Abecarnil.4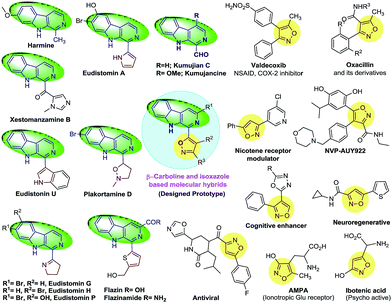 | ||
| Fig. 1 Selected examples of β-carboline- and isoxazole-containing bioactive natural products and drug molecules. | ||
The isoxazole nucleus is represented by a large number of commercial drugs and a few natural products, such as AMPA, ibotenic acid.5 Interestingly, isoxazole derivatives also demonstrate a wide range of biological properties, such as being inhibitors of heat shock protein 90 activity, a broad spectrum of antibiotics, neurone generative agents, antiviral agents, NSAIDs, antiproliferative and immunosuppressive agents (Fig. 1).6 In addition to this, isoxazole derivatives also serve as a latent precursor for the synthesis of enaminones and α-cyanoaldehydes, which in turn act as templates for natural products and other heterocycles, such as quinolines, oxazoles and indoles.7
The excellent activity profile of these pharmacophores motivated us to develop a new molecular hybrid of these two frameworks for our anticancer project. Interestingly, an exponential growth towards the construction of molecular hybrids across the globe has been observed owing to their high affinity and selectivity, better efficacy and a different or dual mode of action.8
The growing resistance against the existing chemotherapeutic agents is another driving force for this paradigm shift towards the development of new molecular hybrids.
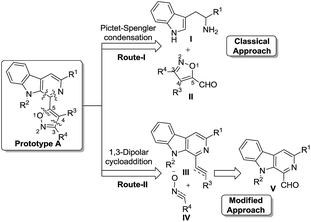
With the objective to synthesize the desired prototype, a retrosynthetic analysis revealed that there may be two possible pathways for the construction of our designed molecular hybrid, as depicted in Fig. 2. The first route involves the conventional approach, where the Pictet–Spengler condensation of tryptamine/L-tryptophan ester (I) with isoxazole-5-carbaldehyde (II), followed by oxidation of the resulting product may furnish the desired prototype.9 However, the synthesis of isoxazole-5-carbaldehyde (II) with substitution at the C-3 and C-4 positions is deemed difficult and there are only limited reports of it available in the literature.10 Furthermore, it is anticipated that steric hindrance offered by the C-3 and C-4 substituents (bulky nature) may hamper the Pictet–Spengler condensation. Alternatively, a more facile and straightforward approach may be 1,3-dipolar cycloaddition between β-carboline-containing dipolarophile (III) and a suitable dipole (IV, nitrile oxide), which can afford the targeted molecules (prototype-A).11 The β-carboline-allied dipolarophiles (III) in turn may be obtained from 1-formyl pyrido[3,4-b]indole derivatives (V). Apart from this, 1-formyl pyrido[3,4-b]indole (V) could also generate 1,3-dipoles, which upon reaction with a suitable dipolarophile may generate the C-3 substituted isoxazole derivatives. It is evident that these approaches have broad scope and advantages over the classical approach as they could yield the products with a high degree of diversity. In this context, we engineered a modified approach, where a versatile precursor in the form of 1-formyl-9H-β-carboline (an alkaloid known as Kumujian C)12 was developed.12f We successfully demonstrated the applicability of this synthon for the synthesis of canthin-6-one, harmicine and homofascaplysin mimics by using Morita–Baylis–Hillman (MBH) chemistry.13 More recently, we were able to fabricate β-carbolines-conjugated imidazo[1,2-a]pyridines and γ-lactones by using this template.14 Additionally, we could generate β-carboline D-ring fused frameworks by the application of intramolecular cycloaddition reactions.15 Notably, other research groups also effectively used this building block for the synthesis of β-carboline-containing natural products, catalysts and anticancer agents.16
An overview of the literature reflected that the cycloaddition of nitrile oxides with dipolarophiles is the best route to access isoxazole derivatives.17 However, thermal cycloaddition offers poor regioselectivity; therefore, various metal-assisted (Cu/Ru/Cr) approaches have been discovered to increase the efficacy of this reaction and to generate high chemo- and regioselectivity.18 Apart from the metal-assisted protocols, a few metal-free approaches using reagents like hypervalent iodine, Et3N, DBU, NHCs and NaOCl/Et3N have been developed.19 Due to the explosive nature and reactivity issues associated with hypervalent iodine reagents, we aimed to develop a cost-effective and benign approach to serve this purpose.20 Since during recent years, much emphasis has been given to the development of sustainable approaches, we therefore directed our efforts towards engineering a metal-free strategy. A deep review of the literature revealed that there was only one report available towards the synthesis of β-carboline-conjugated isoxazoles; however, this was at the C-5 position with only three analogues.21 Additionally, this Pd-assisted approach was only able to produce the products in low yields under refluxing conditions. More importantly, a general approach dedicated to the synthesis of trisubstituted isoxazole-tethered β-carboline-based molecular hybrids has so far remained undeveloped. Therefore, a metal-free, simple, convenient, robust and cost-effective approach was developed and detailed studies were performed to achieve the synthesis of desired prototypes, which are presented herein.
Results and discussion
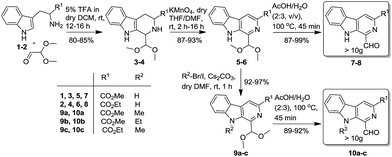
To achieve the synthesis of the designed prototypes, initially 1-formyl-9H-β-carbolines (7–8) and its N-substituted derivatives (10a–c) were prepared from L-tryptophan ester (1–2) by using a recently reported scale-up procedure, as illustrated in Scheme 1.12f In the first phase of study, it was envisaged achieving the synthesis of C-5 substituted isoxazolyl-based molecular hybrids via a 1,3-dipolar cycloaddition approach. Accordingly, β-carboline substituted dipolarophiles (11a–d) were generated in a facile manner via a Wittig reaction of 1-formyl pyrido[3,4-b]indole derivatives (7, 10a–c) with triethyl phosphonoacetate in anhydrous THF.22 Interestingly, the products were obtained in excellent yields (89–97%) and could be collected by simple filtration. The nitrile oxides (A–C) were generated in situ to avoid the dimerization from the corresponding hydroxymoyl chlorides in a two-step process from aromatic carbaldehydes.23 Next, the optimization studies were initiated with (E)-methyl 1-(3-ethoxy-3-oxoprop-1-en-1-yl)-9-methyl-9H-pyrido[3,4-b]indole-3-carboxylate (11a) as the dipolarophile with nitrile oxide A in the presence of Et3N in anhydrous THF (Scheme 2).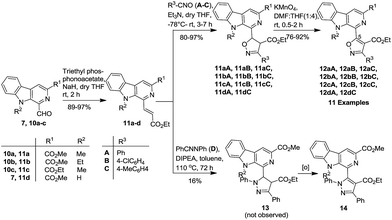 | ||
| Scheme 2 Synthesis of β-carboline–isoxazole- (12) and β-carboline-pyrazole-based molecular hybrids (14). | ||
To attain a high regioselectivity and to avoid the competitive dimerization of nitrile oxide, the cycloaddition reaction was performed at −78 °C under dilute conditions with the slow addition of Et3N to form hydroxymoyl chlorides (A). It was pleasing to note that the nitrile oxides A reacted smoothly with 11a in a regioselective manner to yield the anticipated isoxazoline derivative (11aA) as a single diastereomer. The crystallisation/trituration of the crude product with MeOH afforded the analytically pure product in 92% yield. The optimisation studies for the cycloaddition reaction were also investigated with DABCO and DBU in THF, but offered the product 11aA in only low yields. Interestingly, it was observed that the isoxazoline product (11aA) was stable at low temperature but was prompt to undergo slow aerial oxidation at room temperature, which alleviated our objective. The further oxidation of isoxazoline product (11aA) was achieved in anhydrous THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (4
DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of KMnO4. The oxidation process was very fast and exothermic when the reaction was performed in anhydrous DMF and required only 25 min for completion (80% yield, Table 1, ESI†) but work-up was slightly tedious. However, when THF was used as a solvent, the reaction was completed in 6 h, but simple filtration of the reaction contents through a celite bed, followed by evaporation of the solvent afforded the pure product (84%). Therefore, to take advantage of both, we performed the reaction in a 4
1) in the presence of KMnO4. The oxidation process was very fast and exothermic when the reaction was performed in anhydrous DMF and required only 25 min for completion (80% yield, Table 1, ESI†) but work-up was slightly tedious. However, when THF was used as a solvent, the reaction was completed in 6 h, but simple filtration of the reaction contents through a celite bed, followed by evaporation of the solvent afforded the pure product (84%). Therefore, to take advantage of both, we performed the reaction in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of anhydrous THF and DMF (Table 1,† entry 3), and it yielded the product in a better yield (90%) in 40 min. With these optimized conditions in hand, we further investigated the scope of the reaction with various β-carboline-containing dipolarophiles (11a–c) with different nitrile oxides (A–C). To our pleasure, all the substrates responded positively towards the cycloaddition reaction and smoothly furnished the desired β-carboline-substituted isoxazoline derivatives (11aA–aC, 11bA–bC, 11cA–cC and 11dA–dC). The isoxazoline products (11) were isolated and purified, but immediately subjected to further oxidation with KMnO4 to produce the corresponding β-carboline-conjugated isoxazole derivatives (12aA–aC, 12bA–bC, 12cA–cC and 12dA–dC) in good to excellent yields (76–92%) (Fig. 4), as illustrated in Scheme 2. Encouraged by this success, we planned to expand the scope of strategy for the synthesis of β-carboline and pyrazole-based molecular hybrids (14), as presented in Scheme 2. However, when 11a was treated with nitrile imine (D), a complex mixture of products was obtained. The major product was isolated in a 16% yield and on the basis of the spectroscopic data its structure was confirmed to be an oxidized product (14). We could not isolate compound 13 from the reaction mixture. Though, we attempted to optimize the reaction conditions (Table 2, see ESI†) by varying the solvent and base to obtain the product in a better yield, we did not have much success. It was also observed that nitrile imines were potentially allergic to the skin and were an irritant to the eyes, which halted our efforts in this direction.
1 mixture of anhydrous THF and DMF (Table 1,† entry 3), and it yielded the product in a better yield (90%) in 40 min. With these optimized conditions in hand, we further investigated the scope of the reaction with various β-carboline-containing dipolarophiles (11a–c) with different nitrile oxides (A–C). To our pleasure, all the substrates responded positively towards the cycloaddition reaction and smoothly furnished the desired β-carboline-substituted isoxazoline derivatives (11aA–aC, 11bA–bC, 11cA–cC and 11dA–dC). The isoxazoline products (11) were isolated and purified, but immediately subjected to further oxidation with KMnO4 to produce the corresponding β-carboline-conjugated isoxazole derivatives (12aA–aC, 12bA–bC, 12cA–cC and 12dA–dC) in good to excellent yields (76–92%) (Fig. 4), as illustrated in Scheme 2. Encouraged by this success, we planned to expand the scope of strategy for the synthesis of β-carboline and pyrazole-based molecular hybrids (14), as presented in Scheme 2. However, when 11a was treated with nitrile imine (D), a complex mixture of products was obtained. The major product was isolated in a 16% yield and on the basis of the spectroscopic data its structure was confirmed to be an oxidized product (14). We could not isolate compound 13 from the reaction mixture. Though, we attempted to optimize the reaction conditions (Table 2, see ESI†) by varying the solvent and base to obtain the product in a better yield, we did not have much success. It was also observed that nitrile imines were potentially allergic to the skin and were an irritant to the eyes, which halted our efforts in this direction.
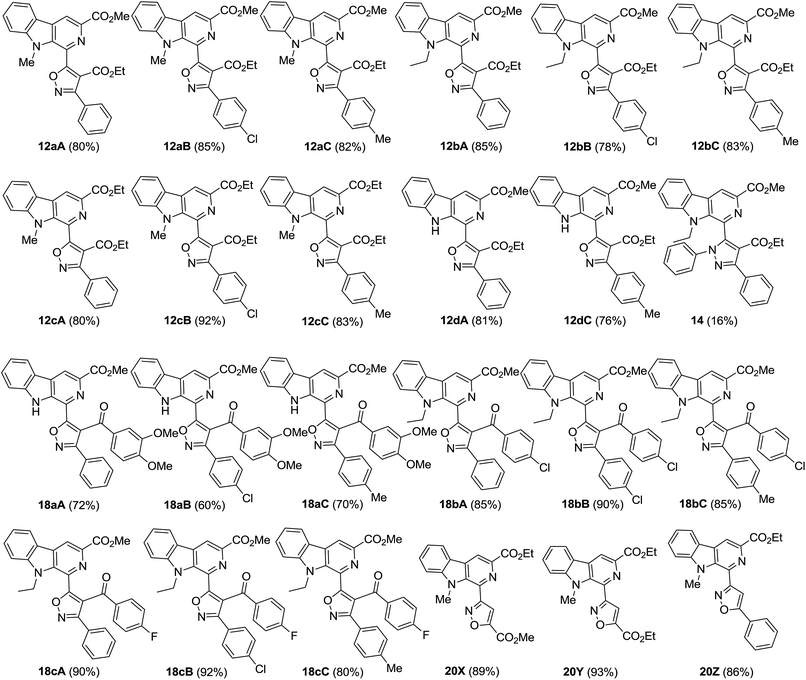 | ||
| Fig. 4 Structures of various β-carboline- (C-5)isoxazole- and (C-3)isoxazole-based molecular hybrids synthesized during the present study. | ||
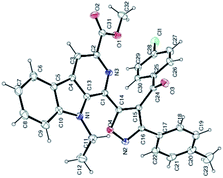
Recently, Chauhan and co-workers demonstrated that β-carboline-based chalcones display potent cytotoxic properties against human breast cancer MCF-7 cell lines, with an IC50 up to 2.25 μM.16d Their findings stimulated us to explore these products as a template (as dipolarophiles) for the synthesis of new β-carboline–isoxazole-based molecular hybrids for our anticancer project. Accordingly, the synthesis of β-carboline-based chalcones (16a–c) was achieved via the Claisen–Schmidt condensation of the 1-formyl-pyrido[3,4-b]indole derivatives (7 and 10b) with different acetophenones (15a–c) having electron donating as well as electron withdrawing substituents, as depicted in Scheme 3. Interestingly, these dipolarophiles (β-carboline-based chalcones, 16a–c) also reacted smoothly with various nitrile oxides (A–C) in the presence of Et3N in anhydrous THF at −78 °C to yield the desired isoxazoline derivatives (17aA–aC, 17bA–bC and 17cA–cC) in 81–93% yields. Interestingly, under these metal-free conditions, complete regioselectivity was obtained, which was further unambiguously assigned on the basis of the single crystal X-ray crystallographic analysis of 18bC (Fig. 3).24 The β-carboline-linked isoxazoline derivatives (17aA–aC, 17bA–bC and 17cA–cC) were subsequently oxidized to the corresponding isoxazole derivatives (18aA–aC, 18bA–bC and 18cA–cC) with KMnO4 within 0.5–3 h and afforded the products (Fig. 4) with high purity in good to excellent yields (60–92%). It is interesting to mention here that no column chromatographic purification was required at any step and that trituration/washing with MeOH furnished the analytically pure product.
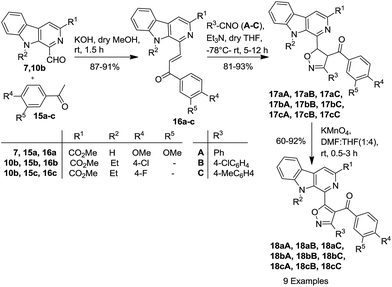 | ||
| Scheme 3 Synthesis of β-carboline–isoxazole molecular hybrids (18) using β-carboline based chalcones as template. | ||
After the successful synthesis of β-carboline-(C-5)isoxazole-based molecular hybrids (12 and 18) from β-carboline-containing dipolarophiles (11 and 16), we directed our efforts to construct β-carboline-(C-3)isoxazole-based molecular hybrids (Scheme 4). It was envisaged that a cycloaddition reaction between a β-carboline-linked 1,3-dipole and a suitable external dipolarophile may fulfil our objective. Accordingly, ethyl 1-formyl-9-methyl-9H-pyrido[3,4-b]indole-3-carboxylate (10c) was treated with NH2OH·HCl to obtain the corresponding oxime (19), which was subsequently reacted with N-chlorosuccinimide (NCS) in anhydrous DMF to yield the corresponding hydroxymoyl chloride (20). Furthermore, the β-carboline-tethered nitrile oxide was generated in situ in the presence of Et3N at −78 °C and reacted with different dipolarophiles (X–Z), which smoothly furnished the desired molecular hybrids (20X–Z) in excellent yields (86–93%), as depicted in Scheme 4. Interestingly, this modified approach also afforded these novel β-carboline-conjugated isoxazole derivatives with complete regioselectivity, as confirmed by spectroscopic analysis. It is anticipated that low temperature, secondary interactions with the carbonyl functionality and steric limitation offered by the β-carboline ring are responsible for the high regioselectivity. Gratifyingly, both the approaches yielded the products with high purity and furthermore, no column chromatographic purification was required at any step.
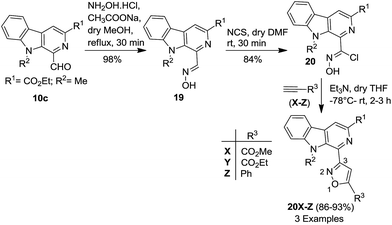 | ||
| Scheme 4 Synthesis of β-carboline-(C-3)isoxazole-based molecular hybrids (20X–Z) using β-carboline-linked dipoles as a template. | ||
Conclusions
In conclusion, we successfully developed a simple, efficient and easy-to-execute metal-free protocol for the synthesis of β-carboline- (C-5)isoxazole- and (C-3)isoxazole-based molecular hybrids via a 1,3-dipolar cycloaddition approach. Unfortunately, the developed strategy was not found to be suitable for the synthesis of β-carboline-(C-5)pyrazole-conjugated derivatives. More importantly, all the products were afforded in high yield and no column chromatographic purification was required at any stage (except for the pyrazole derivative), and analytically pure products were obtained by a simple filtration of the reaction mixture or by recrystallization of the product with MeOH. In brief, this strategy offers an alternative to the possible classical approach and has several advantages, such as operational simplicity, easy purification procedure, wide functional group compatibility and, importantly, unlimited diversity can be introduced in these interesting molecular architectures. The anticancer evaluation and lead optimisation studies are underway and will be described in due course.Experimental
General section
All chemicals and reagents were purchased from Sigma Aldrich, Acros, Avera Synthesis, Spectrochem Pvt. Ltd. and used without further purification. Commercially available anhydrous solvents (THF, DMF, toluene, MeOH and CH2Cl2; Spectrochem) were utilized in the reactions. Thin-layer chromatography (TLC) was performed using pre-coated aluminium plates purchased from E. Merck (silica gel 60 PF254, 0.25 mm). Column chromatography was performed using Spectrochem silica gel (60–120 mesh). Melting points were determined in open capillary tubes on a Precision Digital melting point apparatus (LABCO make) containing silicon oil and the results are uncorrected. IR spectra were recorded using an Agilent FTIR spectrophotometer. 1H NMR and 13C NMR spectra were recorded either on an Avance III Bruker or JEOL JNM-ECS spectrometer at operating frequencies of 200/400 MHz (1H) or 100 MHz (13C) as indicated in the individual spectrum, using TMS as an internal standard. The MS spectra were recorded on a Xevo G2-SQ Tof (Water, USA) or Thermo Finnigan LCQ Advantage Ion Trap Mass Spectrometer. Elemental analyses were performed on a Carlo-Erba 108 or an Elementar Vario EL III microanalyzer. The room temperature varied between 25 °C and 40 °C. The multiplicities in the 1H-NMR spectra are presented as s for singlet, d for doublet, dd for doublet of doublet, t for triplet and m for multiplet.Experimental section
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v)).
20, v/v)).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1705 (CO2CH3 and CO2CH2CH3), 1619 (C
20, v/v); IR (KBr): νmax = 1705 (CO2CH3 and CO2CH2CH3), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1261 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.17 (t, J = 7.2 Hz, 3H), 3.98 (s, 3H), 4.19 (q, J = 7.2 Hz, 2H), 4.35 (s, 3H), 6.56 (q, J = 4.8 Hz, 1H), 7.85 (d, J = 4.4 Hz, 1H), 7.37 (t, J = 7.2 Hz, 1H), 7.40–7.53 (m, 3H), 7.56 (d, J = 8.0 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.93 (d, J = 2.4 Hz, 2H), 8.18 (d, J = 7.6 Hz, 1H), 8.84 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 29.8, 32.7, 52.5, 54.3, 62.2, 84.3, 110.2, 111.4, 118.4, 121.1, 121.3, 121.7, 127.9, 128.6, 130.4, 135.7, 136.9, 131.4, 141.1, 142.8, 156.6, 166.1, 169.7; MS (ES): m/z (%) = 458.2 (100) [M + 1]+; C26H23N3O5 (457.1638): calcd for C 68.26, H 5.07, N 9.19; found C 68.33, H 5.12, N 9.26.
N–O), 1261 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.17 (t, J = 7.2 Hz, 3H), 3.98 (s, 3H), 4.19 (q, J = 7.2 Hz, 2H), 4.35 (s, 3H), 6.56 (q, J = 4.8 Hz, 1H), 7.85 (d, J = 4.4 Hz, 1H), 7.37 (t, J = 7.2 Hz, 1H), 7.40–7.53 (m, 3H), 7.56 (d, J = 8.0 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.93 (d, J = 2.4 Hz, 2H), 8.18 (d, J = 7.6 Hz, 1H), 8.84 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 29.8, 32.7, 52.5, 54.3, 62.2, 84.3, 110.2, 111.4, 118.4, 121.1, 121.3, 121.7, 127.9, 128.6, 130.4, 135.7, 136.9, 131.4, 141.1, 142.8, 156.6, 166.1, 169.7; MS (ES): m/z (%) = 458.2 (100) [M + 1]+; C26H23N3O5 (457.1638): calcd for C 68.26, H 5.07, N 9.19; found C 68.33, H 5.12, N 9.26.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (4
DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (10 mL), KMnO4 (0.60 g) was added portion-wise at room temperature and the reaction mixture stirred for an additional 30 min at room temperature. On completion of the reaction as monitored by TLC, the contents were filtered through a celite bed under vacuum and the bed was washed three times with chloroform. The collected organic layers were combined and concentrated under vacuum to yield a dull white solid product, which was washed twice with anhydrous diethyl ether and then air dried under vacuum to obtain the analytically pure white solid product, 12aA (0.16 g, 80%, Rf = 0.30 (hexane/EtOAc, 80
1, v/v) (10 mL), KMnO4 (0.60 g) was added portion-wise at room temperature and the reaction mixture stirred for an additional 30 min at room temperature. On completion of the reaction as monitored by TLC, the contents were filtered through a celite bed under vacuum and the bed was washed three times with chloroform. The collected organic layers were combined and concentrated under vacuum to yield a dull white solid product, which was washed twice with anhydrous diethyl ether and then air dried under vacuum to obtain the analytically pure white solid product, 12aA (0.16 g, 80%, Rf = 0.30 (hexane/EtOAc, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v)).
20, v/v)).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1703 (CO2CH3 and CO2CH2CH3), 1617 (C
20, v/v); IR (KBr): νmax = 1703 (CO2CH3 and CO2CH2CH3), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.73 (t, J = 7.2 Hz, 3H), 3.68 (s, 3H), 3.98 (q, J = 7.2 Hz, 2H), 4.06 (s, 3H), 7.45–7.55 (m, 5H), 7.67–7.74 (m, 1H), 7.82 (t, J = 7.2 Hz, 2H), 8.29 (d, J = 7.8.0 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.3, 31.2, 52.9, 60.9, 110.0, 118.9, 120.9, 121.4, 121.9, 127.6, 127.8, 128.3, 128.5, 129.1, 129.4, 129.7, 130.2, 130.8, 137.2, 142.9, 160.9, 162.7, 166.0, 170.8 ppm; MS (ES): m/z (%) = 456.1 (100) [M + 1]+; C26H21N3O5 (455.1481): calcd for C 68.56, H 4.65, N 9.23; found C 68.64, H 4.68, N 9.31.
N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.73 (t, J = 7.2 Hz, 3H), 3.68 (s, 3H), 3.98 (q, J = 7.2 Hz, 2H), 4.06 (s, 3H), 7.45–7.55 (m, 5H), 7.67–7.74 (m, 1H), 7.82 (t, J = 7.2 Hz, 2H), 8.29 (d, J = 7.8.0 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.3, 31.2, 52.9, 60.9, 110.0, 118.9, 120.9, 121.4, 121.9, 127.6, 127.8, 128.3, 128.5, 129.1, 129.4, 129.7, 130.2, 130.8, 137.2, 142.9, 160.9, 162.7, 166.0, 170.8 ppm; MS (ES): m/z (%) = 456.1 (100) [M + 1]+; C26H21N3O5 (455.1481): calcd for C 68.56, H 4.65, N 9.23; found C 68.64, H 4.68, N 9.31.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1711 (CO2CH2CH3), 1721 (CO2CH3), 1611 (C
20, v/v); IR (KBr): νmax = 1711 (CO2CH2CH3), 1721 (CO2CH3), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.71 (t, J = 7.1 Hz, 3H), 3.68 (s, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.10 (s, 3H), 7.45 (t, J = 7.7 Hz, 1H), 7.48–7.52 (m, 2H), 7.54 (d, J = 8.4 Hz, 1H), 7.71–7.75 (m, 1H), 7.78–7.82 (m, 2H), 8.29 (d, J = 7.9 Hz, 1H), 9.06 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.4, 31.4, 53.1, 61.2, 110.1, 113.1, 119.1, 121.0, 121.6, 122.1, 126.1, 128.7, 129.9, 130.9, 136.7, 137.3, 142.9, 160.8, 161.9, 166.1, 171.3 ppm; MS (ES): m/z (%) = 490.2 (100) [M + 1]+; C26H20ClN3O5 (489.1091): calcd for C 68.74, H 4.11, N 8.58; found C 68.85, H 4.16, N 8.70.
N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.71 (t, J = 7.1 Hz, 3H), 3.68 (s, 3H), 3.98 (q, J = 7.1 Hz, 2H), 4.10 (s, 3H), 7.45 (t, J = 7.7 Hz, 1H), 7.48–7.52 (m, 2H), 7.54 (d, J = 8.4 Hz, 1H), 7.71–7.75 (m, 1H), 7.78–7.82 (m, 2H), 8.29 (d, J = 7.9 Hz, 1H), 9.06 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.4, 31.4, 53.1, 61.2, 110.1, 113.1, 119.1, 121.0, 121.6, 122.1, 126.1, 128.7, 129.9, 130.9, 136.7, 137.3, 142.9, 160.8, 161.9, 166.1, 171.3 ppm; MS (ES): m/z (%) = 490.2 (100) [M + 1]+; C26H20ClN3O5 (489.1091): calcd for C 68.74, H 4.11, N 8.58; found C 68.85, H 4.16, N 8.70.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1728 (CO2CH3 and CO2CH2CH3), 1610 (C
20, v/v); IR (KBr): νmax = 1728 (CO2CH3 and CO2CH2CH3), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (200 MHz, CDCl3) δ = 1.19 (t, J = 7.1 Hz, 3H), 2.40 (s, 3H), 3.98 (s, 3H), 4.20 (q, J = 7.1 Hz, 2H), 4.34 (s, 3H), 7.24–7.57 (m, 5H), 7.82 (d, J = 8.2 Hz, 2H), 8.18 (d, J = 7.8 Hz, 1H), 8.83 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 21.6, 32.5, 52.4, 61.2, 110.2, 118.3, 121.0, 121.4, 121.7, 126.0, 127.8, 129.2, 129.4, 130.8, 135.4, 137.0, 137.3, 140.6, 142.8, 156.5, 166.4, 169.9 ppm; MS (ES): m/z (%) = 470.1 (100) [M + 1]+, 492.1 (33) [M + 23]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.19, H 5.00, N 9.06.
N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (200 MHz, CDCl3) δ = 1.19 (t, J = 7.1 Hz, 3H), 2.40 (s, 3H), 3.98 (s, 3H), 4.20 (q, J = 7.1 Hz, 2H), 4.34 (s, 3H), 7.24–7.57 (m, 5H), 7.82 (d, J = 8.2 Hz, 2H), 8.18 (d, J = 7.8 Hz, 1H), 8.83 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 21.6, 32.5, 52.4, 61.2, 110.2, 118.3, 121.0, 121.4, 121.7, 126.0, 127.8, 129.2, 129.4, 130.8, 135.4, 137.0, 137.3, 140.6, 142.8, 156.5, 166.4, 169.9 ppm; MS (ES): m/z (%) = 470.1 (100) [M + 1]+, 492.1 (33) [M + 23]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.19, H 5.00, N 9.06.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1710 (CO2CH2CH3), 1725 (CO2CH3) 1617 (C
20, v/v); IR (KBr): νmax = 1710 (CO2CH2CH3), 1725 (CO2CH3) 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.55 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.0 Hz, 3H), 3.89 (q, J = 7.1 Hz, 2H), 3.94 (s, 3H), 4.05–4.19 (m, 2H), 7.46 (t, J = 7.4 Hz, 1H), 7.60 (t, J = 7.1 Hz, 3H), 7.76 (t, J = 7.7 Hz, 1H), 7.83–7.95 (m, 3H), 8.60 (d, J = 7.9 Hz, 1H), 9.22 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 39.6, 53.0, 61.0, 110.3, 113.0, 119.1, 121.4, 121.5, 122.1, 127.7, 128.4, 129.0, 129.5, 129.8, 130.4, 131.1, 136.4, 137.2, 142.0, 160.7, 162.9, 166.1, 171.4 ppm; MS (ES): m/z (%) = 470.2 (100) [M + 1]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.19, H 5.01, N 9.02.
N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.55 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.0 Hz, 3H), 3.89 (q, J = 7.1 Hz, 2H), 3.94 (s, 3H), 4.05–4.19 (m, 2H), 7.46 (t, J = 7.4 Hz, 1H), 7.60 (t, J = 7.1 Hz, 3H), 7.76 (t, J = 7.7 Hz, 1H), 7.83–7.95 (m, 3H), 8.60 (d, J = 7.9 Hz, 1H), 9.22 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 39.6, 53.0, 61.0, 110.3, 113.0, 119.1, 121.4, 121.5, 122.1, 127.7, 128.4, 129.0, 129.5, 129.8, 130.4, 131.1, 136.4, 137.2, 142.0, 160.7, 162.9, 166.1, 171.4 ppm; MS (ES): m/z (%) = 470.2 (100) [M + 1]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.19, H 5.01, N 9.02.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1704 (CO2CH2CH3), 1721 (CO2CH3), 1620 (C
20, v/v); IR (KBr): νmax = 1704 (CO2CH2CH3), 1721 (CO2CH3), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.51 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.0 Hz, 3H), 4.18 (q, J = 7.1 Hz, 2H), 4.28–4.34 (m, 2H), 4.40 (s, 3H), 7.41 (t, J = 7.5 Hz, 1H), 7.50–7.60 (m, 2H), 7.74 (t, J = 7.6 Hz, 1H), 7.81–7.90 (m, 2H), 7.94 (d, J = 6.4 Hz, 1H), 8.50 (d, J = 7.8 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 39.7, 53.0, 61.1, 110.3, 112.9, 119.1, 121.4, 121.6, 122.2, 126.2, 128.8, 129.9, 130.1, 131.2, 136.4, 137.2, 142.0, 160.8, 162.0, 166.1, 171.7 ppm; MS (ES): m/z (%) = 504.2 (100) [M + 1]+; C27H22ClN3O5 (503.1248): calcd for C 64.35, H 4.40, N 8.34; found C 64.44, H 4.43, N 8.40.
N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.51 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.0 Hz, 3H), 4.18 (q, J = 7.1 Hz, 2H), 4.28–4.34 (m, 2H), 4.40 (s, 3H), 7.41 (t, J = 7.5 Hz, 1H), 7.50–7.60 (m, 2H), 7.74 (t, J = 7.6 Hz, 1H), 7.81–7.90 (m, 2H), 7.94 (d, J = 6.4 Hz, 1H), 8.50 (d, J = 7.8 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 39.7, 53.0, 61.1, 110.3, 112.9, 119.1, 121.4, 121.6, 122.2, 126.2, 128.8, 129.9, 130.1, 131.2, 136.4, 137.2, 142.0, 160.8, 162.0, 166.1, 171.7 ppm; MS (ES): m/z (%) = 504.2 (100) [M + 1]+; C27H22ClN3O5 (503.1248): calcd for C 64.35, H 4.40, N 8.34; found C 64.44, H 4.43, N 8.40.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1720 (CO2CH3), 1702 (CO2CH2CH3), 1626 (C
20, v/v); IR (KBr): νmax = 1720 (CO2CH3), 1702 (CO2CH2CH3), 1626 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.71 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 2.45 (s, 3H), 3.97 (q, J = 7.1 Hz, 2H), 4.06 (s, 3H), 4.07–4.13 (m, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 8.3 Hz, 1H), 7.69–7.73 (m, 3H), 8.29 (d, J = 7.8 Hz, 1H), 9.06 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 21.6, 39.6, 53.0, 61.0, 110.3, 113.0, 119.1, 121.3, 121.5, 122.1, 124.6, 129.2, 129.4, 129.8, 131.1, 136.4, 137.1, 140.6, 142.0, 160.9, 162.9, 166.2, 171.2 ppm; MS (ES): m/z (%) = 484.2 (100) [M + 1]+; C28H25N3O5 (483.1794): calcd. for C 69.55, H 5.21, N 8.69; found C 69.66, H 5.25, N 8.77.
N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.71 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H), 2.45 (s, 3H), 3.97 (q, J = 7.1 Hz, 2H), 4.06 (s, 3H), 4.07–4.13 (m, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 8.3 Hz, 1H), 7.69–7.73 (m, 3H), 8.29 (d, J = 7.8 Hz, 1H), 9.06 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.5, 14.1, 21.6, 39.6, 53.0, 61.0, 110.3, 113.0, 119.1, 121.3, 121.5, 122.1, 124.6, 129.2, 129.4, 129.8, 131.1, 136.4, 137.1, 140.6, 142.0, 160.9, 162.9, 166.2, 171.2 ppm; MS (ES): m/z (%) = 484.2 (100) [M + 1]+; C28H25N3O5 (483.1794): calcd. for C 69.55, H 5.21, N 8.69; found C 69.66, H 5.25, N 8.77.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1703 (CO2CH2CH3), 1719 (CO2CH3), 1616 (C
20, v/v); IR (KBr): νmax = 1703 (CO2CH2CH3), 1719 (CO2CH3), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.14 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H), 4.13–4.19 (m, 2H), 4.31 (q, J = 7.1 Hz, 2H), 4.39 (s, 3H), 7.41 (t, J = 7.5 Hz, 1H), 7.50–7.60 (m, 3H), 7.74 (t, J = 7.3 Hz, 1H), 7.81–7.90 (m, 2H), 7.94 (d, J = 6.4 Hz, 1H), 8.50 (d, J = 7.8 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 14.5, 32.4, 54.3, 61.3, 62.1, 110.2, 118.2, 121.0, 121.3, 121.7, 127.9, 128.6, 128.9, 129.1, 130.3, 130.8, 135.7, 136.9, 137.1, 142.8, 156.5, 165.9, 169.7 ppm; MS (ES): m/z (%) = 470.2 (100) [M + 1]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.20, H 5.00, N 9.06.
N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.14 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H), 4.13–4.19 (m, 2H), 4.31 (q, J = 7.1 Hz, 2H), 4.39 (s, 3H), 7.41 (t, J = 7.5 Hz, 1H), 7.50–7.60 (m, 3H), 7.74 (t, J = 7.3 Hz, 1H), 7.81–7.90 (m, 2H), 7.94 (d, J = 6.4 Hz, 1H), 8.50 (d, J = 7.8 Hz, 1H), 9.01 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.1, 14.5, 32.4, 54.3, 61.3, 62.1, 110.2, 118.2, 121.0, 121.3, 121.7, 127.9, 128.6, 128.9, 129.1, 130.3, 130.8, 135.7, 136.9, 137.1, 142.8, 156.5, 165.9, 169.7 ppm; MS (ES): m/z (%) = 470.2 (100) [M + 1]+; C27H23N3O5 (469.1638): calcd for C 69.07, H 4.94, N 8.95; found C 69.20, H 5.00, N 9.06.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1702 (CO2CH2CH3), 1717 (CO2CH3), 1611 (C
20, v/v); IR (KBr): νmax = 1702 (CO2CH2CH3), 1717 (CO2CH3), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.73 (t, J = 7.2 Hz, 3H), 1.48 (t, J = 7.2 Hz, 3H), 3.69 (s, 3H), 3.99 (q, J = 7.2 Hz, 2H), 4.54 (q, J = 7.2 Hz, 2H), 7.42–7.54 (m, 4H), 7.71 (t, J = 8.0 Hz, 1H), 7.80 (d, J = 8.0 Hz, 2H), 8.28 (d, J = 8.0 Hz, 1H), 9.02 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.8, 14.6, 31.4, 61.2, 62.0, 110.1, 113.0, 119.0, 121.0, 121.5, 122.1, 126.1, 128.7, 128.9, 129.9, 130.9, 136.7, 137.3, 137.6, 142.9, 160.9, 161.9, 165.5, 171.3 ppm; MS (ES): m/z (%) = 504.2 (100) [M + 1]+; C27H22ClN3O5 (503.1248): calcd for C 64.35, H 4.40, N 8.34; found C 64.47, H 4.44, N 8.42.
N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 0.73 (t, J = 7.2 Hz, 3H), 1.48 (t, J = 7.2 Hz, 3H), 3.69 (s, 3H), 3.99 (q, J = 7.2 Hz, 2H), 4.54 (q, J = 7.2 Hz, 2H), 7.42–7.54 (m, 4H), 7.71 (t, J = 8.0 Hz, 1H), 7.80 (d, J = 8.0 Hz, 2H), 8.28 (d, J = 8.0 Hz, 1H), 9.02 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.8, 14.6, 31.4, 61.2, 62.0, 110.1, 113.0, 119.0, 121.0, 121.5, 122.1, 126.1, 128.7, 128.9, 129.9, 130.9, 136.7, 137.3, 137.6, 142.9, 160.9, 161.9, 165.5, 171.3 ppm; MS (ES): m/z (%) = 504.2 (100) [M + 1]+; C27H22ClN3O5 (503.1248): calcd for C 64.35, H 4.40, N 8.34; found C 64.47, H 4.44, N 8.42.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1704 (CO2CH2CH3), 1620 (C
20, v/v); IR (KBr): νmax = 1704 (CO2CH2CH3), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.19 (t, J = 7.1 Hz, 3H), 1.44 (t, J = 7.2 Hz, 3H), 2.40 (s, 3H), 4.20 (q, J = 7.1 Hz, 2H), 4.37 (s, 3H), 4.44 (q, J = 7.2 Hz, 2H), 7.24 (s, 2H), 7.38 (t, J = 7.5 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.68 (t, J = 7.4 Hz, 1H), 7.81 (d, J = 8.1 Hz, 2H), 8.20 (d, J = 7.8 Hz, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.6, 14.0, 21.1, 31.9, 53.7, 60.7, 61.6, 83.6, 109.7, 117.6, 120.5, 120.6, 121.1, 125.4, 127.2, 128.7, 128.9, 130.1, 135.0, 136.4, 136.5, 140.2, 142.2, 155.9, 165.2, 169.3 ppm; MS (ES): m/z (%) = 484.1 (100) [M + 1]+; C28H25N3O5 (483.1794): calcd for C 69.55, H 5.21, N 8.69; found C 69.66, H 5.26, N 8.74.
N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.19 (t, J = 7.1 Hz, 3H), 1.44 (t, J = 7.2 Hz, 3H), 2.40 (s, 3H), 4.20 (q, J = 7.1 Hz, 2H), 4.37 (s, 3H), 4.44 (q, J = 7.2 Hz, 2H), 7.24 (s, 2H), 7.38 (t, J = 7.5 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.68 (t, J = 7.4 Hz, 1H), 7.81 (d, J = 8.1 Hz, 2H), 8.20 (d, J = 7.8 Hz, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.6, 14.0, 21.1, 31.9, 53.7, 60.7, 61.6, 83.6, 109.7, 117.6, 120.5, 120.6, 121.1, 125.4, 127.2, 128.7, 128.9, 130.1, 135.0, 136.4, 136.5, 140.2, 142.2, 155.9, 165.2, 169.3 ppm; MS (ES): m/z (%) = 484.1 (100) [M + 1]+; C28H25N3O5 (483.1794): calcd for C 69.55, H 5.21, N 8.69; found C 69.66, H 5.26, N 8.74.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v); IR (KBr): νmax = 3379 (N–H), 1705 (CO2CH3 and CO2CH2CH3), 1623 (C
30, v/v); IR (KBr): νmax = 3379 (N–H), 1705 (CO2CH3 and CO2CH2CH3), 1623 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1269 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.96 (t, J = 7.1 Hz, 3H), 3.95 (s, 3H), 4.25 (q, J = 7.1 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.62 (d, J = 1.9 Hz, 3H), 7.68 (t, J = 7.5 Hz, 1H), 7.79–7.82 (m, 3H), 8.52 (d, J = 7.9 Hz, 1H), 9.14 (s, 1H), 12.38 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.0, 52.5, 62.6, 111.6, 112.6, 118.7, 121.6, 121.8, 127.6, 128.2, 128.9, 129.7, 130.5, 131.2, 134.4, 137.7, 141.2, 161.2, 163.6, 165.8, 167.5 ppm; MS (ES): m/z (%) = 442.1 (100) [M + 1]+; C25H19N3O5 (441.1325): calcd for C 68.02, H 4.34, N 9.52; found C 68.14, H 4.39, N 9.61.
N–O), 1269 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.96 (t, J = 7.1 Hz, 3H), 3.95 (s, 3H), 4.25 (q, J = 7.1 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.62 (d, J = 1.9 Hz, 3H), 7.68 (t, J = 7.5 Hz, 1H), 7.79–7.82 (m, 3H), 8.52 (d, J = 7.9 Hz, 1H), 9.14 (s, 1H), 12.38 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.0, 52.5, 62.6, 111.6, 112.6, 118.7, 121.6, 121.8, 127.6, 128.2, 128.9, 129.7, 130.5, 131.2, 134.4, 137.7, 141.2, 161.2, 163.6, 165.8, 167.5 ppm; MS (ES): m/z (%) = 442.1 (100) [M + 1]+; C25H19N3O5 (441.1325): calcd for C 68.02, H 4.34, N 9.52; found C 68.14, H 4.39, N 9.61.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v); IR (KBr): νmax = 1697 (CO2CH3 and CO2CH2CH3), 1621 (C
30, v/v); IR (KBr): νmax = 1697 (CO2CH3 and CO2CH2CH3), 1621 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1264 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.98 (t, J = 7.1 Hz, 3H), 2.42 (s, 3H), 3.95 (s, 3H), 4.26 (q, J = 7.1 Hz, 2H), 7.38–7.43 (m, 3H), 7.67–7.71 (m, 3H), 7.80 (d, J = 8.2 Hz, 1H), 8.52 (d, J = 7.9 Hz, 1H), 9.14 (s, 1H), 12.40 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ = 12.2, 20.0, 50.9, 60.2, 111.9, 117.5, 119.6, 120.4, 123.4, 126.7, 127.2, 128.1, 129.5, 133.3, 135.2, 139.0, 140.8, 159.5, 161.0, 164.1, 166.5 ppm; MS (ES): m/z (%) = 456.2 (100) [M + 1]+; C26H21N3O5 (455.1481): calcd for C 68.56, H 4.65, N 9.23; found C 68.68, H 4.70, N 9.30.
N–O), 1264 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 0.98 (t, J = 7.1 Hz, 3H), 2.42 (s, 3H), 3.95 (s, 3H), 4.26 (q, J = 7.1 Hz, 2H), 7.38–7.43 (m, 3H), 7.67–7.71 (m, 3H), 7.80 (d, J = 8.2 Hz, 1H), 8.52 (d, J = 7.9 Hz, 1H), 9.14 (s, 1H), 12.40 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ = 12.2, 20.0, 50.9, 60.2, 111.9, 117.5, 119.6, 120.4, 123.4, 126.7, 127.2, 128.1, 129.5, 133.3, 135.2, 139.0, 140.8, 159.5, 161.0, 164.1, 166.5 ppm; MS (ES): m/z (%) = 456.2 (100) [M + 1]+; C26H21N3O5 (455.1481): calcd for C 68.56, H 4.65, N 9.23; found C 68.68, H 4.70, N 9.30.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v)). We wish to mention that even after 3 days the reaction was not complete and 5–6 products were visible in the crude mixture on TLC analysis. We purified the major product from the reaction mixture, which was analysed as 14 (in situ oxidized product).
30, v/v)). We wish to mention that even after 3 days the reaction was not complete and 5–6 products were visible in the crude mixture on TLC analysis. We purified the major product from the reaction mixture, which was analysed as 14 (in situ oxidized product).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v); IR (KBr): νmax = 1703 (CO2CH2CH3), 1718 (CO2CH3); 1H NMR (400 MHz, CDCl3) δ = 0.56 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H), 3.77–3.89 (m, 3H), 4.08 (s, 3H), 4.10–4.16 (m, 1H), 7.17 (d, J = 7.3 Hz, 3H), 7.38 (t, J = 7.5 Hz, 1H), 7.43–7.49 (m, 6H), 7.64 (t, J = 7.5 Hz, 1H), 7.85 (d, J = 7.9 Hz, 2H), 8.23 (d, J = 7.8 Hz, 1H), 8.97 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.0, 14.4, 43.6, 51.8, 61.2, 109.4, 122.4, 122.6, 122.7, 127.6, 128.1, 128.2, 128.3, 128.8, 128.9, 135.5, 137.3, 139.2, 140.0, 141.3, 141.9, 144.3, 160.8, 164.9 ppm; MS (ES): m/z (%) = 545.0 (100) [M + 1]+; C33H28N4O4 (544.2111): calcd for C 72.78, H 5.18, N 10.29; found C 72.90, H 5.22, N 10.36.
30, v/v); IR (KBr): νmax = 1703 (CO2CH2CH3), 1718 (CO2CH3); 1H NMR (400 MHz, CDCl3) δ = 0.56 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H), 3.77–3.89 (m, 3H), 4.08 (s, 3H), 4.10–4.16 (m, 1H), 7.17 (d, J = 7.3 Hz, 3H), 7.38 (t, J = 7.5 Hz, 1H), 7.43–7.49 (m, 6H), 7.64 (t, J = 7.5 Hz, 1H), 7.85 (d, J = 7.9 Hz, 2H), 8.23 (d, J = 7.8 Hz, 1H), 8.97 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.0, 14.4, 43.6, 51.8, 61.2, 109.4, 122.4, 122.6, 122.7, 127.6, 128.1, 128.2, 128.3, 128.8, 128.9, 135.5, 137.3, 139.2, 140.0, 141.3, 141.9, 144.3, 160.8, 164.9 ppm; MS (ES): m/z (%) = 545.0 (100) [M + 1]+; C33H28N4O4 (544.2111): calcd for C 72.78, H 5.18, N 10.29; found C 72.90, H 5.22, N 10.36.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v); IR (KBr): νmax = 3360 (N–H), 1659 (CO), 1710 (CO2CH3), 1621 (C
30, v/v); IR (KBr): νmax = 3360 (N–H), 1659 (CO), 1710 (CO2CH3), 1621 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 3.68 (s, 3H), 3.75 (s, 3H), 3.79 (s, 3H), 6.87 (t, J = 7.6 Hz, 1H), 7.39 (s, 2H), 7.53–7.60 (m, 4H), 7.69 (s, 3H), 7.89 (s, 1H), 8.47 (d, J = 6.6 Hz, 1H), 8.94 (s, 1H), 12.47 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 50.1, 53.9, 54.0, 108.7, 109.1, 111.7, 114.3, 116.6, 119.0, 119.3, 120.2, 123.3, 126.1, 127.3, 127.7, 128.7, 129.2, 132.1, 134.7, 140.4, 147.0, 151.8, 159.4, 163.5, 164.6, 186.3 ppm; MS (ES): m/z (%) = 534.1 (100) [M + 1]+; C31H23N3O6 (533.1587): calcd for C 69.79, H 4.35, N 7.88; found C 69.92, H 4.40, N 8.00.
N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 3.68 (s, 3H), 3.75 (s, 3H), 3.79 (s, 3H), 6.87 (t, J = 7.6 Hz, 1H), 7.39 (s, 2H), 7.53–7.60 (m, 4H), 7.69 (s, 3H), 7.89 (s, 1H), 8.47 (d, J = 6.6 Hz, 1H), 8.94 (s, 1H), 12.47 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 50.1, 53.9, 54.0, 108.7, 109.1, 111.7, 114.3, 116.6, 119.0, 119.3, 120.2, 123.3, 126.1, 127.3, 127.7, 128.7, 129.2, 132.1, 134.7, 140.4, 147.0, 151.8, 159.4, 163.5, 164.6, 186.3 ppm; MS (ES): m/z (%) = 534.1 (100) [M + 1]+; C31H23N3O6 (533.1587): calcd for C 69.79, H 4.35, N 7.88; found C 69.92, H 4.40, N 8.00.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v); IR (KBr): νmax = 3472 (N–H), 1720 (CO2CH3), 1672 (CO), 1619 (C
40, v/v); IR (KBr): νmax = 3472 (N–H), 1720 (CO2CH3), 1672 (CO), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 3.94 (s, 3H), 4.03 (s, 3H), 4.11 (s, 3H), 7.13 (d, J = 8.5 Hz, 1H), 7.29 (d, J = 8.6 Hz, 2H), 7.34–7.39 (m, 1H), 7.57 (d, J = 8.6 Hz, 2H), 7.62 (d, J = 3.9 Hz, 2H), 7.78 (d, J = 1.7 Hz, 1H), 8.18 (d, J = 7.9 Hz, 1H), 8.85 (s, 1H), 9.01 (d, J = 7.1 Hz, 1H), 9.70 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 52.7, 56.1, 56.2, 110.1, 110.6, 111.0, 112.5, 118.0, 121.2, 121.8, 126.5, 126.8, 128.3, 128.7, 129.0, 129.2, 129.5, 130.5, 135.1, 136.6, 136.7, 140.9, 149.5, 154.7, 157.4, 166.3, 193.8 ppm; MS (ES): m/z (%) = 568.1 (100) [M + 1]+; C31H22ClN3O6 (567.1197): calcd for C 65.55, H 3.90, N 7.40; found C 65.67, H 3.94, N 7.49.
N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 3.94 (s, 3H), 4.03 (s, 3H), 4.11 (s, 3H), 7.13 (d, J = 8.5 Hz, 1H), 7.29 (d, J = 8.6 Hz, 2H), 7.34–7.39 (m, 1H), 7.57 (d, J = 8.6 Hz, 2H), 7.62 (d, J = 3.9 Hz, 2H), 7.78 (d, J = 1.7 Hz, 1H), 8.18 (d, J = 7.9 Hz, 1H), 8.85 (s, 1H), 9.01 (d, J = 7.1 Hz, 1H), 9.70 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 52.7, 56.1, 56.2, 110.1, 110.6, 111.0, 112.5, 118.0, 121.2, 121.8, 126.5, 126.8, 128.3, 128.7, 129.0, 129.2, 129.5, 130.5, 135.1, 136.6, 136.7, 140.9, 149.5, 154.7, 157.4, 166.3, 193.8 ppm; MS (ES): m/z (%) = 568.1 (100) [M + 1]+; C31H22ClN3O6 (567.1197): calcd for C 65.55, H 3.90, N 7.40; found C 65.67, H 3.94, N 7.49.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v); IR (KBr): νmax = 3457 (N–H),1722 (CO2CH3), 1669 (CO), 1617 (C
40, v/v); IR (KBr): νmax = 3457 (N–H),1722 (CO2CH3), 1669 (CO), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1256 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 2.31 (s, 3H), 3.69 (s, 3H), 3.85 (s, 3H), 3.97 (s, 3H), 7.10 (d, J = 8.6 Hz, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.35 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 1.4 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.75 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 9.03 (s, 1H), 12.21 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 21.5, 52.6, 56.2, 56.3, 110.6, 111.1, 112.4, 117.8, 121.1, 121.2, 121.8, 125.4, 126.5, 127.4, 128.5, 129.6, 130.4, 135.0, 136.7, 140.8, 141.1, 141.5, 149.5, 154.5, 158.2, 166.4, 194.0 ppm; MS (ES): m/z (%) = 548.1 (100) [M + 1]+; C32H25N3O6 (547.1743): calcd for C 70.19, H 4.60, N 7.67; found C 70.31, H 4.65, N 7.76.
N–O), 1256 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 2.31 (s, 3H), 3.69 (s, 3H), 3.85 (s, 3H), 3.97 (s, 3H), 7.10 (d, J = 8.6 Hz, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.35 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 1.4 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.75 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 8.4 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 9.03 (s, 1H), 12.21 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 21.5, 52.6, 56.2, 56.3, 110.6, 111.1, 112.4, 117.8, 121.1, 121.2, 121.8, 125.4, 126.5, 127.4, 128.5, 129.6, 130.4, 135.0, 136.7, 140.8, 141.1, 141.5, 149.5, 154.5, 158.2, 166.4, 194.0 ppm; MS (ES): m/z (%) = 548.1 (100) [M + 1]+; C32H25N3O6 (547.1743): calcd for C 70.19, H 4.60, N 7.67; found C 70.31, H 4.65, N 7.76.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1719 (CO2CH3), 1667 (CO), 1615 (C
20, v/v); IR (KBr): νmax = 1719 (CO2CH3), 1667 (CO), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1248 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.36 (t, J = 7.0 Hz, 3H), 3.80 (s, 3H), 4.47 (q, J = 7.0 Hz, 2H), 7.27 (d, J = 7.8 Hz, 2H), 7.42–7.51 (m, 4H), 7.66 (d, J = 5.9 Hz, 2H), 7.74 (t, J = 8.1 Hz, 3H), 7.88 (d, J = 7.8 Hz, 1H), 8.52 (d, J = 7.5 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.3, 110.6, 118.8, 119.0, 121.4, 121.6, 122.0, 127.7, 128.5, 128.7, 129.0, 129.9, 130.5, 131.2, 132.0, 135.7, 136.3, 136.9, 140.0, 142.3, 165.8, 169.0, 188.5 ppm; MS (ES): m/z (%) = 536.1 (100) [M + 1]+; C31H22ClN3O4 (535.1299): calcd for C 69.47, H 4.14, N 7.84; found C 69.60, H 4.20, N 7.91.
N–O), 1248 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.36 (t, J = 7.0 Hz, 3H), 3.80 (s, 3H), 4.47 (q, J = 7.0 Hz, 2H), 7.27 (d, J = 7.8 Hz, 2H), 7.42–7.51 (m, 4H), 7.66 (d, J = 5.9 Hz, 2H), 7.74 (t, J = 8.1 Hz, 3H), 7.88 (d, J = 7.8 Hz, 1H), 8.52 (d, J = 7.5 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.3, 110.6, 118.8, 119.0, 121.4, 121.6, 122.0, 127.7, 128.5, 128.7, 129.0, 129.9, 130.5, 131.2, 132.0, 135.7, 136.3, 136.9, 140.0, 142.3, 165.8, 169.0, 188.5 ppm; MS (ES): m/z (%) = 536.1 (100) [M + 1]+; C31H22ClN3O4 (535.1299): calcd for C 69.47, H 4.14, N 7.84; found C 69.60, H 4.20, N 7.91.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1672 (CO), 1716 (CO2CH3), 1620 (C
20, v/v); IR (KBr): νmax = 1672 (CO), 1716 (CO2CH3), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.37 (t, J = 6.9 Hz, 3H), 3.81 (s, 3H), 4.46 (q, J = 6.9 Hz, 2H), 7.25 (d, J = 8.3 Hz, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.59 (d, J = 8.3 Hz, 2H), 7.71 (d, J = 8.2 Hz, 4H), 7.77 (t, J = 7.4 Hz, 1H), 7.88 (d, J = 8.3 Hz, 1H), 8.52 (d, J = 7.8 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 118.7, 118.8, 121.4, 121.7, 122.0, 126.1, 127.4, 128.7, 129.3, 129.8, 130.0, 131.1, 132.1, 135.7, 136.2, 136.8, 136.9, 140.1, 142.3, 161.2, 165.7, 169.1, 188.4 ppm; MS (ES): m/z (%) = 570.2 (100) [M + 1]+; C31H21Cl2N3O4 (569.0909): calcd for C 65.27, H 3.71, N 7.37; found C 65.33, H 3.72, N 7.40.
N–O), 1263 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.37 (t, J = 6.9 Hz, 3H), 3.81 (s, 3H), 4.46 (q, J = 6.9 Hz, 2H), 7.25 (d, J = 8.3 Hz, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.59 (d, J = 8.3 Hz, 2H), 7.71 (d, J = 8.2 Hz, 4H), 7.77 (t, J = 7.4 Hz, 1H), 7.88 (d, J = 8.3 Hz, 1H), 8.52 (d, J = 7.8 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 118.7, 118.8, 121.4, 121.7, 122.0, 126.1, 127.4, 128.7, 129.3, 129.8, 130.0, 131.1, 132.1, 135.7, 136.2, 136.8, 136.9, 140.1, 142.3, 161.2, 165.7, 169.1, 188.4 ppm; MS (ES): m/z (%) = 570.2 (100) [M + 1]+; C31H21Cl2N3O4 (569.0909): calcd for C 65.27, H 3.71, N 7.37; found C 65.33, H 3.72, N 7.40.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1672 (CO), 1716 (CO2CH3), 1622 (C
20, v/v); IR (KBr): νmax = 1672 (CO), 1716 (CO2CH3), 1622 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.36 (t, J = 7.0 Hz, 3H), 2.35 (s, 3H), 3.97 (s, 3H), 4.48 (q, J = 7.0 Hz, 2H), 7.25–7.32 (m, 4H), 7.44 (t, J = 7.5 Hz, 1H), 7.55 (d, J = 8.0 Hz, 2H), 7.69–7.79 (m, 3H), 7.89 (d, J = 8.4 Hz, 1H), 8.52 (d, J = 7.7 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.5, 110.6, 118.8, 119.0, 121.4, 121.6, 122.0, 124.7, 127.7, 128.3, 128.7, 129.7, 129.9, 131.2, 131.9, 135.8, 136.3, 136.9, 139.9, 140.8, 142.3, 162.0, 165.8, 168.8, 188.7 ppm; MS (ES): m/z (%) = 550.1 (100) [M + 1]+. C33H24ClN3O4 (549.1455): calcd for C 69.88, H 4.40, N 7.64; found C 70.01, H 4.45, N 7.73.
N–O), 1262 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.36 (t, J = 7.0 Hz, 3H), 2.35 (s, 3H), 3.97 (s, 3H), 4.48 (q, J = 7.0 Hz, 2H), 7.25–7.32 (m, 4H), 7.44 (t, J = 7.5 Hz, 1H), 7.55 (d, J = 8.0 Hz, 2H), 7.69–7.79 (m, 3H), 7.89 (d, J = 8.4 Hz, 1H), 8.52 (d, J = 7.7 Hz, 1H), 9.00 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.5, 110.6, 118.8, 119.0, 121.4, 121.6, 122.0, 124.7, 127.7, 128.3, 128.7, 129.7, 129.9, 131.2, 131.9, 135.8, 136.3, 136.9, 139.9, 140.8, 142.3, 162.0, 165.8, 168.8, 188.7 ppm; MS (ES): m/z (%) = 550.1 (100) [M + 1]+. C33H24ClN3O4 (549.1455): calcd for C 69.88, H 4.40, N 7.64; found C 70.01, H 4.45, N 7.73.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1667 (CO), 1716 (CO2CH3), 1616 (C
20, v/v); IR (KBr): νmax = 1667 (CO), 1716 (CO2CH3), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1269 (Ar-C–O)isoxazole; 1H NMR (200 MHz, CDCl3) δ = 1.47 (t, J = 7.2 Hz, 3H), 3.85 (s, 3H), 4.41 (q, J = 7.2 Hz, 2H), 6.93 (t, J = 8.6 Hz, 2H), 7.38–7.45 (m, 4H), 7.59–7.70 (m, 4H), 7.84–7.91 (m, 2H), 8.22 (d, J = 7.8 Hz, 1H), 8.90 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 115.5, 115.7, 118.8, 119.2, 121.5, 121.6, 122.1, 127.7, 127.8, 128.5, 129.0, 129.9, 130.5, 131.9, 132.5, 132.6, 133.7, 136.4, 136.9, 142.3, 162.1, 164.7, 165.9, 167.2, 168.9, 188.2 ppm; MS (ES): m/z (%) = 520.2 (100) [M + 1]+; C31H22FN3O4 (519.1594): calcd for C 71.67, H 4.27, N 8.09; found C 71.76, H 4.31, N 8.16.
N–O), 1269 (Ar-C–O)isoxazole; 1H NMR (200 MHz, CDCl3) δ = 1.47 (t, J = 7.2 Hz, 3H), 3.85 (s, 3H), 4.41 (q, J = 7.2 Hz, 2H), 6.93 (t, J = 8.6 Hz, 2H), 7.38–7.45 (m, 4H), 7.59–7.70 (m, 4H), 7.84–7.91 (m, 2H), 8.22 (d, J = 7.8 Hz, 1H), 8.90 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 115.5, 115.7, 118.8, 119.2, 121.5, 121.6, 122.1, 127.7, 127.8, 128.5, 129.0, 129.9, 130.5, 131.9, 132.5, 132.6, 133.7, 136.4, 136.9, 142.3, 162.1, 164.7, 165.9, 167.2, 168.9, 188.2 ppm; MS (ES): m/z (%) = 520.2 (100) [M + 1]+; C31H22FN3O4 (519.1594): calcd for C 71.67, H 4.27, N 8.09; found C 71.76, H 4.31, N 8.16.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1665 (CO), 1712 (CO2CH3), 1615 (C
20, v/v); IR (KBr): νmax = 1665 (CO), 1712 (CO2CH3), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.48 (t, J = 7.1 Hz, 3H), 3.86 (s, 3H), 4.42 (q, J = 7.1 Hz, 2H), 6.94 (t, J = 8.6 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.42 (t, J = 7.8 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.72 (t, J = 7.7 Hz, 1H), 7.85–7.89 (m, 2H), 8.22 (d, J = 7.8 Hz, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 115.5, 115.7, 118.9, 121.4, 121.7, 122.1, 126.2, 127.4, 129.3, 129.8, 130.0, 132.0, 132.4, 132.5, 136.8, 136.9, 142.3, 161.2, 165.8, 169.0, 188.0 ppm; MS (ES): m/z (%) = 554.1 (100) [M + 1]+; C31H21ClFN3O4 (553.1205): calcd for C 67.21, H 3.82, N 7.59; found C 67.32, H 3.86, N 7.67.
N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.48 (t, J = 7.1 Hz, 3H), 3.86 (s, 3H), 4.42 (q, J = 7.1 Hz, 2H), 6.94 (t, J = 8.6 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.42 (t, J = 7.8 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.72 (t, J = 7.7 Hz, 1H), 7.85–7.89 (m, 2H), 8.22 (d, J = 7.8 Hz, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 40.4, 52.6, 110.6, 115.5, 115.7, 118.9, 121.4, 121.7, 122.1, 126.2, 127.4, 129.3, 129.8, 130.0, 132.0, 132.4, 132.5, 136.8, 136.9, 142.3, 161.2, 165.8, 169.0, 188.0 ppm; MS (ES): m/z (%) = 554.1 (100) [M + 1]+; C31H21ClFN3O4 (553.1205): calcd for C 67.21, H 3.82, N 7.59; found C 67.32, H 3.86, N 7.67.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1667 (CO), 1718 (CO2CH3), 1620 (C
20, v/v); IR (KBr): νmax = 1667 (CO), 1718 (CO2CH3), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1265 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.47 (t, J = 7.2 Hz, 3H), 2.36 (s, 3H), 3.84 (s, 3H), 4.41 (q, J = 7.2 Hz, 2H), 6.94 (t, J = 8.6 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 7.5 Hz, 1H), 7.55 (d, J = 8.2 Hz, 3H), 7.71 (t, J = 7.5 Hz, 1H), 7.87–7.90 (m, 2H), 8.22 (d, J = 7.9 Hz, 1H), 8.86 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 21.6, 40.3, 52.6, 110.6, 115.5, 115.7, 118.8, 121.4, 121.6, 122.1, 124.7, 127.8, 128.4, 129.7, 129.8, 131.9, 132.5, 132.6, 133.7, 136.9, 140.8, 142.3, 162.0, 164.7, 165.9, 167.2, 168.7, 188.3 ppm; MS (ES): m/z (%) = 534.1 (100) [M + 1]+; C32H24FN3O4 (533.1751): calcd for C 72.04, H 4.53, N 7.88; found C 72.13, H 4.57, N 7.94.
N–O), 1265 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.47 (t, J = 7.2 Hz, 3H), 2.36 (s, 3H), 3.84 (s, 3H), 4.41 (q, J = 7.2 Hz, 2H), 6.94 (t, J = 8.6 Hz, 2H), 7.19 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 7.5 Hz, 1H), 7.55 (d, J = 8.2 Hz, 3H), 7.71 (t, J = 7.5 Hz, 1H), 7.87–7.90 (m, 2H), 8.22 (d, J = 7.9 Hz, 1H), 8.86 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.4, 21.6, 40.3, 52.6, 110.6, 115.5, 115.7, 118.8, 121.4, 121.6, 122.1, 124.7, 127.8, 128.4, 129.7, 129.8, 131.9, 132.5, 132.6, 133.7, 136.9, 140.8, 142.3, 162.0, 164.7, 165.9, 167.2, 168.7, 188.3 ppm; MS (ES): m/z (%) = 534.1 (100) [M + 1]+; C32H24FN3O4 (533.1751): calcd for C 72.04, H 4.53, N 7.88; found C 72.13, H 4.57, N 7.94.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v); IR (KBr): νmax = 1699 (CO2CH2CH3), 3451 (NOH); 1H NMR (400 MHz, CDCl3) δ = 1.50 (t, J = 7.1 Hz, 3H), 4.07 (s, 3H), 4.25 (s, 1H), 4.53 (q, J = 7.1 Hz, 2H), 7.37 (t, J = 7.5 Hz, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 8.18 (d, J = 7.8 Hz, 1H), 8.84 (s, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.5, 33.7, 62.1, 110.3, 118.6, 121.2, 121.9, 122.0, 130.2, 132.0, 133.8, 134.9, 137.6, 143.1, 148.6, 164.4 ppm; MS (ES): m/z (%) = 298.3 (100) [M + 1]+; C16H15N3O3 (297.1113): calcd for C 64.64, H 5.09, N 14.13; found C 64.70, H 5.11, N 14.17.
30, v/v); IR (KBr): νmax = 1699 (CO2CH2CH3), 3451 (NOH); 1H NMR (400 MHz, CDCl3) δ = 1.50 (t, J = 7.1 Hz, 3H), 4.07 (s, 3H), 4.25 (s, 1H), 4.53 (q, J = 7.1 Hz, 2H), 7.37 (t, J = 7.5 Hz, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 8.18 (d, J = 7.8 Hz, 1H), 8.84 (s, 1H), 8.85 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.5, 33.7, 62.1, 110.3, 118.6, 121.2, 121.9, 122.0, 130.2, 132.0, 133.8, 134.9, 137.6, 143.1, 148.6, 164.4 ppm; MS (ES): m/z (%) = 298.3 (100) [M + 1]+; C16H15N3O3 (297.1113): calcd for C 64.64, H 5.09, N 14.13; found C 64.70, H 5.11, N 14.17.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v)).
20, v/v)).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1741 (CO2CH3), 1717 (CO2CH2CH3), 1630 (C
20, v/v); IR (KBr): νmax = 1741 (CO2CH3), 1717 (CO2CH2CH3), 1630 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.38 (t, J = 7.1 Hz, 3H), 3.74 (s, 3H), 3.99 (s, 3H), 4.42 (q, J = 7.1 Hz, 2H), 7.43 (s, 1H), 7.76 (s, 2H), 7.82 (s, 1H), 8.56 (s, 1H), 9.13 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.1, 31.6, 51.6, 59.8, 109.3, 110.1, 116.7, 119.3, 119.8, 120.4, 128.3, 128.9, 129.6, 135.0, 135.7, 141.7, 155.4, 158.6, 161.2, 163.5 ppm; MS (ES): m/z (%) = 380.1 (100) [M + 1]+; C20H17N3O5 (379.1168): calcd for C 63.32, H 4.52, N 11.08; found C 63.43, H 4.57, N 11.16.
N–O), 1259 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.38 (t, J = 7.1 Hz, 3H), 3.74 (s, 3H), 3.99 (s, 3H), 4.42 (q, J = 7.1 Hz, 2H), 7.43 (s, 1H), 7.76 (s, 2H), 7.82 (s, 1H), 8.56 (s, 1H), 9.13 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 13.1, 31.6, 51.6, 59.8, 109.3, 110.1, 116.7, 119.3, 119.8, 120.4, 128.3, 128.9, 129.6, 135.0, 135.7, 141.7, 155.4, 158.6, 161.2, 163.5 ppm; MS (ES): m/z (%) = 380.1 (100) [M + 1]+; C20H17N3O5 (379.1168): calcd for C 63.32, H 4.52, N 11.08; found C 63.43, H 4.57, N 11.16.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1754 (CO2CH3), 1716 (CO2CH2CH3), 1627 (C
20, v/v); IR (KBr): νmax = 1754 (CO2CH3), 1716 (CO2CH2CH3), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1257 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.39 (t, J = 6.8 Hz, 6H), 3.75 (s, 3H), 4.44 (q, J = 7.1 Hz, 4H), 7.44 (t, J = 7.1 Hz, 1H), 7.75 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 8.0 Hz, 1H), 8.56 (d, J = 7.5 Hz, 1H), 9.13 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 14.0, 14.4, 32.7, 61.1, 62.4, 111.1, 111.6, 118.4, 120.4, 121.2, 122.4, 129.8, 130.5, 136.2, 136.7, 142.9, 156.3, 160.1, 162.5, 164.9, 175.0 ppm; MS (ES): m/z (%) = 394.2 (100) [M + 1]+; C21H19N3O5 (393.1325): calcd for C 64.12, H 4.87, N 10.68; found C 64.23, H 4.90, N 10.74.
N–O), 1257 (Ar-C–O)isoxazole; 1H NMR (400 MHz, DMSO-d6) δ = 1.39 (t, J = 6.8 Hz, 6H), 3.75 (s, 3H), 4.44 (q, J = 7.1 Hz, 4H), 7.44 (t, J = 7.1 Hz, 1H), 7.75 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 8.0 Hz, 1H), 8.56 (d, J = 7.5 Hz, 1H), 9.13 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 14.0, 14.4, 32.7, 61.1, 62.4, 111.1, 111.6, 118.4, 120.4, 121.2, 122.4, 129.8, 130.5, 136.2, 136.7, 142.9, 156.3, 160.1, 162.5, 164.9, 175.0 ppm; MS (ES): m/z (%) = 394.2 (100) [M + 1]+; C21H19N3O5 (393.1325): calcd for C 64.12, H 4.87, N 10.68; found C 64.23, H 4.90, N 10.74.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); IR (KBr): νmax = 1717 (CO2CH2CH3), 1628 (C
20, v/v); IR (KBr): νmax = 1717 (CO2CH2CH3), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.50 (t, J = 7.1 Hz, 3H), 3.96 (s, 3H), 4.55 (q, J = 7.1 Hz, 2H), 7.22 (s, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.49–7.57 (m, 4H), 7.70 (t, J = 7.4 Hz, 1H), 7.91–7.93 (dd, J1 = 7.8 Hz, J2 = 8.3 Hz, 2H), 8.27 (d, J = 7.8 Hz, 1H), 8.97 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.6, 32.2, 61.8, 101.9, 110.4, 118.0, 121.1, 121.2, 121.4, 121.8, 126.1, 127.4, 129.2, 129.5, 129.7, 130.5, 131.2, 132.1, 136.9, 137.4, 143.4, 162.7, 165.8, 170.2 ppm; MS (ES): m/z (%) = 398.1 (100) [M + 1]+; C24H19N3O3 (397.1426): calcd for C 72.53, H 4.82, N 10.57; found C 72.60, H 4.88, N 10.66.
N–O), 1260 (Ar-C–O)isoxazole; 1H NMR (400 MHz, CDCl3) δ = 1.50 (t, J = 7.1 Hz, 3H), 3.96 (s, 3H), 4.55 (q, J = 7.1 Hz, 2H), 7.22 (s, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.49–7.57 (m, 4H), 7.70 (t, J = 7.4 Hz, 1H), 7.91–7.93 (dd, J1 = 7.8 Hz, J2 = 8.3 Hz, 2H), 8.27 (d, J = 7.8 Hz, 1H), 8.97 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ = 14.6, 32.2, 61.8, 101.9, 110.4, 118.0, 121.1, 121.2, 121.4, 121.8, 126.1, 127.4, 129.2, 129.5, 129.7, 130.5, 131.2, 132.1, 136.9, 137.4, 143.4, 162.7, 165.8, 170.2 ppm; MS (ES): m/z (%) = 398.1 (100) [M + 1]+; C24H19N3O3 (397.1426): calcd for C 72.53, H 4.82, N 10.57; found C 72.60, H 4.88, N 10.66.Acknowledgements
D. S., N. D. and V. K. acknowledge Science and Engineering Research Board (SERB)-Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and Ministry of Human Resource Development (MHRD), New Delhi, India for Junior and Senior Research Fellowships. V. S. and B. S. Kaith gratefully acknowledges the financial support in the form of research grant from CSIR (02(0202)/14/EMR-II), DST (CS-361/2011), and DST-FIST (CSI-228/2011) New Delhi (India). Central Facility, Indian Institute of Technology Ropar and Central Instrumental Facility, GJ University of Science and Technology, Hisar is gratefully acknowledged for recording MS, elemental analysis and NMR spectra reported in this paper. V. S. and co-workers gratefully acknowledges the motivation and guidance provided by Prof. Diwan S. Rawat, University of Delhi, Delhi.Notes and references
- (a) E. C. Barnes, R. Kumar and R. A. Davis, Nat. Prod. Rep., 2016, 33, 372–381 RSC; (b) H. M. R. Hoffmann and J. Rabe, Angew. Chem., Int. Ed., 1985, 24, 94–110 CrossRef; (c) U. R. Abdelmohsen, K. Bayer and U. Hentschel, Nat. Prod. Rep., 2014, 31, 381–399 RSC; (d) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2013, 30, 694–752 RSC; (e) M. Ishikura, K. Yamada and T. Abe, Nat. Prod. Rep., 2010, 27, 1630–1680 RSC; (f) T. A. Mansoor, C. Ramalhete, J. Molnar, S. Mulhovo and M. J. U. Ferreira, J. Nat. Prod., 2009, 72, 1147–1150 CrossRef CAS PubMed; (g) M. Frederich, M. Tits and L. Angenot, Trans. R. Soc. Trop. Med. Hyg., 2008, 102, 1089–1094 CrossRef PubMed; (h) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2015, 32, 1389–1471 RSC.
- (a) H. Chen, P. Gao, M. Zhang, W. Liao and J. Zhang, New J. Chem., 2014, 38, 4155–4166 RSC; (b) S. U. Dighe, S. Khan, I. Soni, P. Jain, S. Shukla, R. Yadav, P. Sen, S. M. Meeran and S. Batra, J. Med. Chem., 2015, 58, 3485–3499 CrossRef CAS PubMed; (c) A. Kamal, M. P. N. Rao, P. Swapna, V. Srinivasulu, C. Bagul, A. B. Shaik, K. Mullagiri, J. Kovvuri, V. S. Reddy, K. Vidyasagar and N. Nagesh, Org. Biomol. Chem., 2014, 12, 2370–2387 RSC; (d) S. Wang, K. Fang, G. Dong, S. Chen, N. Liu, Z. Miao, J. Yao, J. Li, W. Zhang and C. Sheng, J. Med. Chem., 2015, 58, 6678–6696 CrossRef CAS PubMed; (e) H. Du, H. Gu, N. Li and J. Wang, Med. Chem. Commun., 2016, 7, 636–645 RSC; (f) A. Daugan, P. Grondin, C. Ruault, A.-C. L. M. de Gouville, H. Coste, J. Kirilovsky, F. Hyafil and R. Labaudiniere, J. Med. Chem., 2003, 46, 4525–4532 CrossRef CAS PubMed.
- R. Cao, W. Peng, Z. Wang and A. Xung, Curr. Med. Chem., 2007, 14, 479–500 CrossRef CAS PubMed , and references cited therein.
- (a) L. A. Sorbera, L. Martin, P. A. Leeson and J. Castaner, Drugs Future, 2001, 26, 15–19 CrossRef CAS; (b) A. Daugan, P. Grondin, C. Ruault, A.-C. L. M. de Gouville, H. Coste, J. Kirilovsky, F. Hyafil and R. Labaudiniere, J. Med. Chem., 2003, 46, 4525–4532 CrossRef CAS PubMed; (c) L. Turski, D. N. Stephens, L. H. Jensen, E. N. Peterson, B. S. Meldrum, S. Patel, J. B. Hansen, W. Loscher, H. H. Schneider and R. Schmiechen, J. Pharmacol. Exp. Ther., 1990, 253, 344–352 CAS; (d) G. N. Maw, C. M. Allerton, E. Gbekor and W. A. Million, Bioorg. Med. Chem. Lett., 2003, 13, 1425–1428 CrossRef CAS PubMed.
- (a) P. Krogsgaard-Larsen and J. J. Hansen, in Excitatory Amino Acid Receptors: Design of Agonists and Antagonists, Taylor & Francis, 1992 Search PubMed; (b) Wantanabe, in Pharmacological Research on Traditional Herbal Medicines, CRC Press, 1999 Search PubMed.
- (a) M. J. Drysdale, B. W. Dymock, H. Finch, P. Webb, E. Mcdonald, K. E. James, K. M. Cheung and T. P. Mathews, WO2004072051 A1, 2004; (b) D. Petrik, Y. Jiang, S. G. Birnbaum, C. M. Powell, M. S. Kim, J. Hsieh and A. J. Eisch, FASEB J., 2012, 26, 3148–3162 CrossRef CAS PubMed; (c) K. K. Madsen, R. P. Clausen, O. M. Larsson, P. Krogsgaard-Larsen, A. Schousboe and H. S. White, J. Neurochem., 2002, 109, 139–144 CrossRef PubMed; (d) G. Miranda-Novales, B. E Leanos-Miranda, M. Vilchis-Perez and F. Solorzano-Santos, Ann. Clin. Microbiol. Antimicrob., 2006, 25, 1–5 Search PubMed; (e) G. DiPasquale, C. Rassaret, R. Richter, P. Welaj, J. Gingold and R. Singer, Agents Actions, 1975, 5, 256–263 CrossRef CAS PubMed; (f) S. Sanders and V. Harisdangkul, Am. J. Med. Sci., 2002, 323, 190–193 CrossRef PubMed; (g) K. K. Madsen, B. Ebert, R. P. Clausen, P. Krogsgaard-Larsen, A. Schousboe and H. S. White, J. Pharmacol. Exp. Ther., 2011, 338, 214–219 CrossRef CAS PubMed; (h) J. Yong, C. Lu and X. Wu, Med. Chem. Commun., 2014, 5, 968–972 RSC; (i) A. Kamal, V. S. Reddy, A. B. Shaik, G. B. Kumar, M. V. P. S. Vishnuvardhan, S. Polepalli and N. Jain, Org. Biomol. Chem., 2015, 13, 3416–3431 RSC; (j) A. Kamal, A. B. Shaik, B. B. Rao, I. Khan, G. B. Kumara and N. Jain, Org. Biomol. Chem., 2015, 13, 3416–3431 RSC; (k) J. J. Talley, S. R. Bertenshaw, D. L. Brown, J. S. Carter, M. J. Graneto, M. S. Kellogg, C. M. Koboldt, J. H. Yuan, Y. Y. Zhang and K. Seibert, J. Med. Chem., 2000, 43, 1661–1663 CrossRef CAS PubMed; (l) S. Velaparthi, M. Brunsteiner, R. Uddin, B. Wan, S. G. Franzblau and P. A. Petukhov, J. Med. Chem., 2008, 51, 1999–2002 CrossRef CAS PubMed; (m) X. Hou, J. Zhu, B.-C. Chen, S. H. Watterson, W. J. Pitts, A. J. Dyckman, P. H Carter, A. Mathur and H. Zhang, Org. Process Res. Dev., 2016, 20, 989–995 CrossRef CAS.
- (a) K. C. Coffman, T. A. Palazzo, T. P. Hartley, J. C. Fettinger, D. J. Tantillo and M. J. Kurth, Org. Lett., 2013, 15, 2062–2065 CrossRef CAS PubMed; (b) V. Singh, G. P. Yadav, P. R. Maulik and S. Batra, Synthesis, 2006, 12, 1995–2004 Search PubMed; (c) G. Casnati, A. Quilico, A. Ricca and P. V. Finzi, Tetrahedron Lett., 1966, 7, 233–238 CrossRef; (d) I. Thomson and K. B. G. Torssell, Acta Chem. Scand., Ser. B, 1988, 42, 309–313 CrossRef; (e) S. Jensen and K. B. G. Torssell, Acta Chem. Scand., 1995, 49, 53–56 CrossRef CAS; (f) R. C. F. Jones, A. Chatterley, R. Marty, W. M. Owtonb and M. R. J. Elsegood, Chem. Commun., 2015, 51, 1112–1115 RSC; (g) M. A. Kalwat, Z. Huang, C. Wichaidit, K. McGlynn, S. Earnest, C. Savoia, E. M. Dioum, J. W. Schneider, M. R. Hutchison and M. H. Cobb, ACS Chem. Biol., 2016, 11, 1128–1136 CrossRef CAS PubMed.
- (a) C. Viegas-Junior, A. Danuello, B. V. da Silva, E. J. Barreiro and C. A. Fraga, Curr. Med. Chem., 2007, 14, 1829–1852 CrossRef CAS PubMed; (b) C. Vilanova, S. Díaz-Oltra, J. Murga, E. Falomir, M. Carda, M. Redondo-Horcajo, J. F. Díaz, I. Barasoain and J. A. Marco, J. Med. Chem., 2014, 57, 10391–10403 CrossRef CAS PubMed; (c) R. Oliveira, R. C. Guedes, P. Meireles, I. S. Albuquerque, L. M. Gonçalves, E. Pires, M. R. Bronze, J. Gut, P. J. Rosenthal, M. Prudêncio, R. Moreira, P. M. O'Neill and F. Lopes, J. Med. Chem., 2014, 57, 4916–4923 CrossRef CAS PubMed; (d) S. Manohar, U. C. Rajesh, S. I. Khan, B. L. Tekwani and D. S. Rawat, ACS Med. Chem. Lett., 2012, 3, 555–559 CrossRef CAS PubMed; (e) F. Bellot, F. Coslédan, L. Vendier, J. Brocard, B. Meunier and A. Robert, J. Med. Chem., 2010, 53, 4103–4109 CrossRef CAS PubMed; (f) K. A. Kumar, J. J. La Clair and P. L. Fuchs, Org. Lett., 2011, 13, 5334–5337 CrossRef CAS PubMed; (g) L. A. Graham, J. Suryadi, T. K. West, G. L. Kucera and U. Bierbach, J. Med. Chem., 2012, 55, 7817–7827 CrossRef CAS PubMed; (h) H. Wei, J. Ruan and X. Zhang, RSC Adv., 2016, 6, 10846–10860 RSC; (i) S. Manohar, A. Pepe, C. E. V. Gerena, B. Zayas, S. V. Malhotra and D. S. Rawat, RSC Adv., 2014, 4, 7062–7067 RSC; (j) R. Goel, V. Luxami and K. Paul, RSC Adv., 2015, 5, 37887–37895 RSC; (k) A. Kamal, M. P. N. Rao, P. Swapna, V. Srinivasulu, C. Bagul, A. B. Shaik, K. Mullagiri, J. Kovvuri, V. S. Reddy, K. Vidyasagarb and N. Nagesh, Org. Biomol. Chem., 2014, 12, 2370–2387 RSC; (l) A. Mishra, B. B. Mishra and V. K. Tiwari, RSC Adv., 2015, 5, 41520–41535 RSC.
- (a) A. Pictet and T. Spengler, Ber. Dtsch. Chem. Ges., 1911, 44, 2030–2036 CrossRef CAS; (b) E. D. Cox and J. Cook, Chem. Rev., 1995, 95, 1797–1842 CrossRef CAS; (c) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311–2352 CrossRef CAS PubMed.
- F. Fariña, M. T. Fraile, M. R. Martin, M. V. Martín and A. M. Guereñu, Heterocycles, 1995, 40, 285–292 CrossRef.
- (a) D. V. Vorobyeva, N. M. Karimova, I. L. Odinets, G.-V. Roschenthaler and S. N. Osipov, Org. Biomol. Chem., 2011, 9, 7335–7342 RSC; (b) S. Nagaraju, N. Satyanarayana, B. Paplal, A. K. Vasu, S. Kanvah, B. Sridhar, P. Sripadid and D. Kashinath, RSC Adv., 2015, 5, 94474–94478 RSC; (c) M. L. McIntosh, M. R. Naffziger, B. O. Ashburn, L. N. Zakharovb and R. G. Carter, Org. Biomol. Chem., 2012, 10, 9204–9213 RSC; (d) G. Pal, S. Paul, P. P. Ghosh and A. R. Das, RSC Adv., 2014, 4, 8300–8307 RSC.
- (a) A. Jordaan, L. M. Du Plessis and V. P. Joynt, J. S. Afr. Chem. Inst., 1968, 21, 22–23 CAS; (b) G. Gong, Q. Lin, J. Xu, F. Ye, L. Jiang, W. Liu, M.-F. He, F. Feng, W. Qu and N. Xie, RSC Adv., 2016, 6, 9484–9494 RSC; (c) K. G. Brahmbhatt, N. Ahmed, S. Sabde, D. Mitra, I. P. Singh and K. K. Bhutani, Bioorg. Med. Chem. Lett., 2010, 20, 4416–4419 CrossRef CAS PubMed; (d) M. R. D. Giudice, F. Gatta and G. Settimj, J. Heterocycl. Chem., 1990, 27, 967–973 CrossRef; (e) P. Molina, P. M. Fresneda and S. Garcia-Zafra, Tetrahedron Lett., 1996, 37, 9353–9356 CrossRef CAS; (f) V. Singh, S. Hutait and S. Batra, Eur. J. Org. Chem., 2009, 6211–6216 CrossRef CAS.
- (a) V. Singh, S. Hutait and S. Batra, Eur. J. Org. Chem., 2010, 3684–3691 CrossRef CAS; (b) V. Singh and S. Batra, Curr. Org. Synth., 2012, 9, 513–528 CrossRef CAS; (c) S. Hutait, S. Biswas and S. Batra, Eur. J. Org. Chem., 2012, 2453–2462 CrossRef CAS.
- N. Devi, D. Singh, Honey, S. Mor, S. Chaudhary, R. K. Rawal, V. Kumar, A. K. Chowdhury and V. Singh, RSC Adv., 2016, 6, 43881–43891 RSC.
- (a) S. Hutait, V. Singh and S. Batra, Eur. J. Org. Chem., 2010, 6269–6276 CrossRef CAS; (b) V. Singh, S. Hutait, S. Biswas and S. Batra, Eur. J. Org. Chem., 2010, 531–539 CrossRef CAS.
- (a) S. Ramesh and R. Nagarajan, J. Org. Chem., 2013, 78, 545–558 CrossRef CAS PubMed; (b) S. Ramesh and R. Nagarajan, Tetrahedron, 2013, 69, 4890–4898 CrossRef CAS; (c) S. S. Chauhan, S. Pandey, R. Shivahare, K. Ramalingam, S. Krishna, P. Vishwakarma, M. I. Siddiqi, S. Gupta, N. Goyal and P. M. S. Chauhan, Med. Chem. Commun., 2015, 6, 351–356 RSC; (d) S. S. Chauhan, A. K. Singh, S. Meena, M. Lohani, A. Singh, R. K. Arya, S. H. Cheruvu, J. Sarkar, J. R. Gayen, D. Datta and P. M. S. Chauhan, Bioorg. Med. Chem. Lett., 2014, 24, 2820–2824 CrossRef CAS PubMed; (e) B. Bai, L. Shen, J. Ren and H. J. Zhu, Adv. Synth. Catal., 2012, 354, 354–358 CrossRef CAS.
- (a) M. P. Bourbeau and J. T. Rider, Org. Lett., 2006, 8, 3679–3680 CrossRef CAS PubMed; (b) L. Cecchi, F. De Sarlo and F. Machetti, Eur. J. Org. Chem., 2006, 4852–4860 CrossRef CAS; (c) C.-Y. Chen, T. Andreani and H. Li, Org. Lett., 2011, 13, 6300–6303 CrossRef CAS PubMed; (d) J. A. Crossley and D. L. Browne, J. Org. Chem., 2010, 75, 5414–5416 CrossRef CAS PubMed; (e) E. Gayon, O. Quinonero, S. Lemouzy, E. Vrancken and J.-M. Campagne, Org. Lett., 2011, 13, 6418–6421 CrossRef CAS PubMed; (f) O. Jackowski, T. Lecourt and L. Micouin, Org. Lett., 2011, 13, 5664–5667 CrossRef CAS PubMed; (g) S. Minakata, S. Okumura, T. Nagamachi and Y. Takeda, Org. Lett., 2011, 13, 2966–2969 CrossRef CAS PubMed; (h) M. S. Mohamed Ahmed, K. Kobayashi and A. Mori, Org. Lett., 2005, 7, 4487–4489 CrossRef PubMed; (i) C. Praveen, A. Kalyanasundaram and P. T. Perumal, Synlett, 2010, 777–781 CAS; (j) S.-R. Sheng, X.-L. Liu, Q. Xu and C.-S. Song, Synthesis, 2003, 2763–2764 CrossRef CAS; (k) B. J. Stokes, C. V. Vogel, L. K. Urnezis, M. Pan and T. G. Driver, Org. Lett., 2010, 12, 2884–2887 CrossRef CAS PubMed; (l) S. Tang, J. He, Y. Sun, L. He and X. She, Org. Lett., 2009, 11, 3982–3985 CrossRef CAS PubMed; (m) M. Ueda, A. Sato, Y. Ikeda, T. Miyoshi, T. Naito and O. Miyata, Org. Lett., 2010, 12, 2594–2597 CrossRef CAS PubMed; (n) J. P. Waldo and R. C. Larock, J. Org. Chem., 2007, 72, 9643–9647 CrossRef CAS PubMed; (o) B. Willy, F. Rominger and T. J. J. Müller, Synthesis, 2008, 293–303 CAS.
- (a) C.-K. Chanda, S. Rej and M. H. Huang, Nanoscale, 2013, 5, 12494–12501 RSC; (b) S. B. Bharate, A. K. Padala, B. A. Dar, R. R. Yadav, B. Singh and R. A. Vishwakarma, Tetrahedron Lett., 2013, 54, 3558–3561 CrossRef CAS; (c) T. V. Hansen, P. Wu and V. V. Fokin, J. Org. Chem., 2005, 70, 7761–7764 CrossRef CAS PubMed; (d) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2004, 127, 210–216 CrossRef PubMed.
- (a) A. Yoshimura, K. R. Middleton, A. D. Todora, B. J. Kastern, S. R. Koski, A. V. Maskaev and V. V. Zhdankin, Org. Lett., 2013, 15, 4010–4013 CrossRef CAS PubMed; (b) A. Yoshimura, C. Zhu, K. R. Middleton, A. D. Todora, B. J. Kastern, A. V. Maskaev and V. V. Zhdankin, Chem. Commun., 2013, 49, 4800–4802 RSC; (c) B. A. Mendelsohn, S. Lee, S. Kim, F. Teyssier, V. S. Aulakh and M. A. Ciufolini, Org. Lett., 2009, 11, 1539–1542 CrossRef CAS PubMed; (d) A. M. Jawalekar, E. Reubsaet, F. P. J. T. Rutjes and F. L. van Delft, Chem. Commun., 2011, 47, 3198–3200 RSC; (e) S. Kankala, R. Vadde and C. S. Vasam, Org. Biomol. Chem., 2011, 9, 7869–7876 RSC; (f) S. Mohammed, R. A. Vishwakarma and S. B. Bharate, RSC Adv., 2015, 5, 3470–3473 RSC; (g) A. M. Jawalekar, E. Reubsaet, F. P. J. T. Rutjes and F. L. van Delft, Chem. Commun., 2011, 47, 3198–3200 RSC.
- R. D. Richardson, J. M. Zayed, S. Altermann, D. Smith and T. Wirth, Angew. Chem., Int. Ed., 2007, 46, 6529–6653 CrossRef CAS PubMed.
- T. Tremmel and F. Bracher, Tetrahedron, 2015, 71, 4640–4646 CrossRef CAS.
- G. C. Condie and J. Bergman, Eur. J. Org. Chem., 2004, 1286–1297 CrossRef CAS.
- (a) V. Singh, G. P. Yadav, P. R. Maulik and S. Batra, Tetrahedron, 2008, 64, 2979–2991 CrossRef CAS; (b) V. Singh, V. Singh and S. Batra, Eur. J. Org. Chem., 2008, 5446–5460 CrossRef CAS.
- The single crystal XRD deposition number is CCDC 1485761.†.
Footnote |
| † Electronic supplementary information (ESI) available: General information and NMR spectra (1H and 13C) of new compounds. CCDC 1485761. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra15875g |
| This journal is © The Royal Society of Chemistry 2016 |