Can chiral P(III) coordinate Eu(III)? Unexpected solvent dependent circularly polarised luminescence of BINAP and Eu(III)(hfa)3 in chloroform and acetone†
Yuki Konoa,
Kazuki Nakabayashia,
Sayaka Kitamuraa,
Motohiro Shizumab,
Michiya Fujiki*c and
Yoshitane Imai*a
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Kindai University, 3-4-1 Kowakae, Higashi-Osaka, Osaka 577-8502, Japan. E-mail: y-imai@apch.kindai.ac.jp
bDepartment of Biochemistry, Osaka Municipal Technical Research Institute, 1-6-50 Morinomiya, Joto-ku, Osaka 536-8553, Japan
cGraduate School of Materials Science, Nara Institute of Science and Technology, Takayama, Ikoma, Nara 630-0192, Japan. E-mail: fujikim@ms.naist.jp
First published on 5th April 2016
Abstract
The coordination of Eu(III) (from Eu(III)(hfa)3) by chiral P(III) (from BINAP) led to circularly polarised luminescence in dilute solutions on the order of |gem| ≈ 10−2 at 5D0 → 7F1 and |gem| ≈ 10−3 at 5D0 → 7F2 transitions regardless of the coordinating ability of the solvent (i.e., in non-coordinating ethanol-free chloroform and coordinating acetone).
Introduction
According to an early comprehensive review by Fryzuk et al., alkylated P(III) compounds can coordinate trivalent lanthanoid ions, several actinoid ions and the first transition metal ions.1 Although alkylated and arylated P(III) compounds commonly coordinate second and third transition metals (e.g., Ag(I), Pd(II), Rh(I), Ir(I), and Ru(II)), arylated P(III) compounds that can coordinate lanthanoid ions are rare.2 The most accepted theory describing hard and soft acids and bases (HSAB theory)3 has inspired most researchers in coordination chemistry: hard bases prefer hard acids, while soft bases prefer soft acids. Because Eu(III) has a highly localized positive charge with a small ionic radius (2.31 pm), Eu(III) is preferentially coordinated by O, N, and F as hard base ligands, whereas the interaction between Eu(III) and soft base ligands such as P(III) and S(II) is disfavoured.An open question is whether arylated P(III) species can coordinate Eu(III) as well as second and third transition metals. To address this question, we chose optically inactive Eu(III)(hfa)3(H2O)2 [hfa; 1,1,1,5,5,5-hexafluoro-pentane-1,4-dione, Fig. 1] and 2,2-bis-(diphenylphosphino)-1,1-binaphtha-lene as arylated P(III) (BINAP, Fig. 1). For comparison, 2,2-bis(diphenyl-phosphinyl)-1,1-bi-naphthalene as a typical arylated phosphine(V) oxide was examined (BINAPO, Fig. 1).
Notably, the synthesis and photoluminescence (PL) of optically inactive Eu(III)(hfa)3, Nd(III)(hfa)3 and Sm(III)(hfa)3 with arylated phosphine(V) oxide derivatives as ligands were previously reported.4 Moreover, several chiral Eu(III) complexes could be coordinated by BINAPO and other arylated phosphine(V) oxides exhibited circularly polarised luminescence (CPL) and PL.5 Here, the oxygen atom(s) of phosphine(V) oxide coordinates Eu(III). Noted that two lone pairs of oxygen from BINAPO coordinate Eu(III), whilst one lone pair of phosphine from BINAP was assumed to coordinate Eu(III), as illustrated in Fig. 1. To our knowledge, spectroscopic (CPL, PL, circular dichroism (CD), UV-vis) studies of chiral P(III) compounds and Eu(III) have not been reported to date.
Herein, we evaluated the spectroscopic properties of BINAP upon coordination of Eu(III) by P(III). This new chiral luminophore was obtained by mixing (R)-BINAP (or (S)-BINAP) with Eu(III)(hfa)3(H2O)2 (Fig. 1) in EtOH-free, dehydrated CHCl3 (with amylene as a stabiliser) and dry acetone (Fig. 1). This solvent system was selected to avoid the unfavourable coordination by the lone pair of oxygen in EtOH and water. Notably, ordinary CHCl3 usually contains 1 wt% (≈10−1 M) of EtOH as a stabilizer. This is a large excess to Eu(hfa)3 in our preparation and measurement conditions [(0.1–5) × 10−4 M].
Experimental
General methods
Chiral binaphthyl compounds, (R)-BINAP, (S)-BINAP, (R)-BINAPO and (S)-BINAPO, were purchased from Daicel Corporation (Osaka, Japan). These compounds were purified by recrystallisation from special grade CHCl3 (≈1% EtOH, Wako). Eu(III)(hfa)3 was prepared according to a previously reported method.5b Elemental analysis (average values of three runs); obsd (%). C 21.83, H 0.96. Calcd for C15H7EuF18O8 (Eu(III)(hfa)3(H2O)2) (%). C 22.27, H 0.87; calcd for C15H9EuF18O9 (Eu(III)(hfa)3(H2O)3) (%). C 21.78, H 1.10. According to elemental analysis, Eu(III)(hfa)3 may exist as an adduct with three water molecules rather two water molecules.Measurement of photoluminescence
The PL spectra and absolute PL quantum yields were measured using an Absolute PL Quantum Yield Measurement System (C9920-02, Hamamatsu Photonics, Hamamatsu, Japan) in air at room temperature. The excitation wavelength in acetone solution was 300 nm. EtOH-free dehydrated chloroform (Wako, Osaka, Japan) and spectroscopic grade acetone (Dojindo, Spectrosol, Kumamoto, Japan) were used for spectroscopic measurements.Measurement of CPL spectra
The CPL spectra were obtained with a JASCO (Tokyo, Japan) CPL-200 spectrofluoropolarimeter at room temperature. A scattering angle of 0° was used for the excitation of non-polarised monochromatic incident light, with a bandwidth of 10 nm. Scanning speed was 50 nm per min and time constant of PMT was 8 s. The CPL and PL spectra were smoothed by 4 and 8 time accumulations without numerical smoothing. EtOH-free dehydrated chloroform (Wako) and spectroscopic grade acetone (Dojindo, Spectrosol) were used for spectroscopic measurements. The excitation wavelength in acetone solution (path length 1 mm) was 300 nm. The excitation wavelength in chloroform solution (path length 1 mm) was 300, 325, and 335 nm.Measurement of CD and UV absorption spectra
The CD and UV absorption spectra of the compounds in chloroform and acetone solution states were measured using a JASCO J-820 spectropolarimeter at room temperature. The path length in solution measurement was 1 mm. EtOH-free dehydrated chloroform (Wako) and spectroscopic grade acetone (Dojindo, Spectrosol) were used for spectroscopic measurements.Mass spectral measurements
Mass spectra were taken on an LCMS (liquid chromatography mass spectrometer)-ion trap (IT)-TOF (time of flight) spectrometer (Shimadzu, Kyoto, Japan) with an electrospray ionization source with/without sodium triflate. All mass spectra were measured under the instrumental conditions: solvent, acetonitrile; mass range, m/z 100–2000; spray voltage, 4.5 kV (positive mode) and −3.5 kV (negative mode); nebulizer gas flow rate; 1.5 L min−1; CDL temperature, 100 °C; heat block temperature, 100 °C; ion source pressure, 80 Pa; ion trap pressure, 1.7 × 10−2 Pa; TOF pressure, 1.2 × 10−4 Pa; resolution, >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Calibration and tuning of the instrument were carried out using an available standard solution of trifluoroacetic acid (Shimadzu).
000. Calibration and tuning of the instrument were carried out using an available standard solution of trifluoroacetic acid (Shimadzu).
Results and discussion
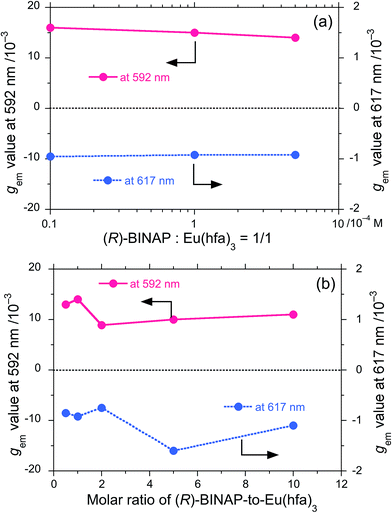
In EtOH-free CHCl3, (R)-BINAP–Eu(III)(hfa)3 (R-Eu) and (S)-BINAP–Eu(III)(hfa)3 (S-Eu) (5.0 × 10−4 M) clearly exhibited CPL and PL, as shown in Fig. 2a (blue and red lines, respectively). PL maxima (λem) appeared at 592, 617, 657, 688 and 703 nm, which are characteristic 4f–4f transitions of Eu(III). The CPL spectra of R-Eu and S-Eu were nearly mirror-images, owing to the chirality of BINAP.In order to evaluate the photoexcited (gem) and ground state chirality (gabs) experimentally, we used gem = 2(IL − IR)/(IL + IR) and gabs = 2(εL − εR)/(εL + εR) = Δε/ε. The gem values of R-Eu in CHCl3 were +1.4 × 10−2 at 592 nm (5D0 → 7F1), −9.2 × 10−4 at 617 nm (5D0 → 7F2), +4.1 × 10−3 at 657 nm (5D0 → 7F3), −4.1 × 10−3 at 688 nm (5D0 → 7F4), and +2.5 × 10−3 at 703 nm (5D0 → 7F4). A CPL band at 586 nm (5D0 → 7F0) was not obvious. The gem values at 592 and 617 nm were almost unchanged when [R-Eu] was in the range of (0.1–5) × 10−4 M in EtOH-free CHCl3 (Fig. 3a, Fig. S1a and b in ESI†). Also, the gem values associated were almost unchanged when the molar ratio of BINAP to Eu(III)(hfa)3 was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 10
2 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a fixed concentration of Eu(hfa)3 at 5 × 10−4 M in EtOH-free CHCl3 (Fig. 3b, Fig. S2a–d in ESI†).
1 with a fixed concentration of Eu(hfa)3 at 5 × 10−4 M in EtOH-free CHCl3 (Fig. 3b, Fig. S2a–d in ESI†).
Similarly, the gabs value and CD/UV-vis spectral profiles were almost unchanged when the molar ratio of BINAP to Eu(III)(hfa)3 was varied from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a fixed concentration of Eu(hfa)3 at 5 × 10−4 M in EtOH-free CHCl3 (Fig. S3a–e in ESI†). These results indicate that P(III) strongly coordinated Eu(III)(hfa)3 and BINAP is a useful chiral ligand for Eu(III)-based CPL materials.
1 with a fixed concentration of Eu(hfa)3 at 5 × 10−4 M in EtOH-free CHCl3 (Fig. S3a–e in ESI†). These results indicate that P(III) strongly coordinated Eu(III)(hfa)3 and BINAP is a useful chiral ligand for Eu(III)-based CPL materials.
For comparison, the CPL/PL spectra of R-Eu and S-Eu that were prepared by mixing equimolar amounts of BINAP and Eu(III)(hfa)3 in acetone are shown in Fig. 2b. Notably, acetone and acetone-d6 are routinely used to prepare and measure various phosphine(V) oxides due to its ability to solubilise several Eu(III) complexes.4,5 The values of λem appeared at 592 nm (5D0 → 7F1) and 612 nm (5D0 → 7F2), with a PL quantum yield (ΦPL) value of 17%. Surprisingly, solvent dependent CPL spectra of R-Eu and S-Eu were observed, although in acetone R-Eu and S-Eu clearly exhibited CPL signals at the 5D0 → 7F2 and 5D0 → 7F1 bands, while the PL spectra in acetone were nearly identical to those in CHCl3 (Fig. 2a).
More surprisingly, in acetone the PL bands of R-Eu and S-Eu at 612 nm resulted in a couplet-like CPL band. The PL band (5D0 → 7F1) at 592 nm resulted in couplet-like CPL bands as well. For R-Eu, the gem values at 5D0 → 7F1 were −4.4 × 10−3 (583 nm) and +4.4 × 10−3 (592 nm), whereas those at the 5D0 → 7F2 band were +1.7 × 10−3 (611 nm) and −1.9 × 10−3 (619 nm). The gem value at 5D0 → 7F4 was −0.7 × 10−3 (701 nm), although the CPL signal at 5D0 → 7F3 (653 nm) was not obvious.
We are aware of the fact that, from a comparison of Fig. 2a and b, the |gem| value of 5D0 → 7F2 transition in acetone (1.9 × 10−3 at 617 nm) is large twice compared to that in CHCl3 (0.9 × 10−3 at 619 nm). Further coordination of O element from solvent acetone is possible associated with two P elements due to BINAP and three hfa ligands. Thus, totally nine coordination may cause a more distorted environment at Eu(III), thus, providing a larger |gem| value.
Next, we investigated whether the couplet-like CPL signal resulted from coordination of Eu(III) by acetone. We measured the CPL/PL spectra of (R)- and/or (S)-BINAPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu(hfa)3 = 1
Eu(hfa)3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with [Eu(III)] = 5 × 10−4 M in EtOH-free chloroform and acetone (Fig. 4a and b). In chloroform, the non-couplet-like CPL spectral profiles were similar to those of R-Eu and S-Eu. In acetone, the couplet-like CPL spectra were similar to those of R-Eu and S-Eu. In chloroform, the gem values of (R)-BINAPO
1 with [Eu(III)] = 5 × 10−4 M in EtOH-free chloroform and acetone (Fig. 4a and b). In chloroform, the non-couplet-like CPL spectral profiles were similar to those of R-Eu and S-Eu. In acetone, the couplet-like CPL spectra were similar to those of R-Eu and S-Eu. In chloroform, the gem values of (R)-BINAPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu (=1
Eu (=1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were +2.0 × 10−2 (590 nm) and −1.4 × 10−3 (617 nm), whereas the gem values for (S)-BINAPO
1) were +2.0 × 10−2 (590 nm) and −1.4 × 10−3 (617 nm), whereas the gem values for (S)-BINAPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu (=1
Eu (=1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were nearly the same absolute values of those of the (R)-complex, albeit with the opposite sign.
1) were nearly the same absolute values of those of the (R)-complex, albeit with the opposite sign.
Similarly, in acetone, (R)-BINAPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu(hfa)3 (=1
Eu(hfa)3 (=1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited similar absolute values at gem = −4.9 × 10−3 (577 nm), +4.8 × 10−3 (593 nm), +2.5 × 10−3 (608 nm) and −2.9 × 10−3 (622 nm), suggesting that the degree of chiral distortion at Eu(III) induced by two chiral O elements of BINAPO is almost identical to that of two chiral P elements in BINAP, perhaps because the binaphthyl ring has rotational freedom along the C–C single bond of the C2-symmetric Naph–Naph.
1) exhibited similar absolute values at gem = −4.9 × 10−3 (577 nm), +4.8 × 10−3 (593 nm), +2.5 × 10−3 (608 nm) and −2.9 × 10−3 (622 nm), suggesting that the degree of chiral distortion at Eu(III) induced by two chiral O elements of BINAPO is almost identical to that of two chiral P elements in BINAP, perhaps because the binaphthyl ring has rotational freedom along the C–C single bond of the C2-symmetric Naph–Naph.
The intense coordination of Eu(III) by the P(III) of BINAP was further supported by LC-IT-TOF-MS analysis, which was carried out in the negative and positive modes (Fig. S4 and S5 in ESI†). The magnified negative mode MS data (Fig. S4,† bottom) clearly showed two natural isotopes of 151Eu(III) and 153Eu(III), that include single (R)-BINAP and hfa (m/z = 978.82 and 980.82), in addition to (R)-BINAP (m/z = 660.89) and hfa (m/z = 206.99). However, regardless of negative- and positive-mode electrospray MS measurements by adding sodium triflate, no parent peaks of R-Eu (m/z = 1396.08 and 1398.08) with Na+ (m/z = 22.99) or triflate (m/z = 148.95) without H2O (m/z = 18.01) and two/three H2O molecules as adducts were detected (Fig. S6 and S7 in ESI†). Thus, clear evidence of species comprised of (R)-BINAP and Eu(hfa)3 was not obtained. The coordination of Eu(III) by O (from hfa) is considerably weak and the Eu–O bonds easily dissociate under laser-assisted ionization energy, while the Eu(III)–P(III) bond is considerably strong regardless of the hard acid and soft base relationship.
Pristine Eu(III)(hfa)3 might exist as a mixture of Δ- and Λ-forms, as well as a tridendate Ir(III) complex.6 In the absence of oxygen-containing molecules, chiral P(III) preferentially coordinates to Δ-Eu(III) (or Λ-Eu(III)), due to the non-coordinating CHCl3. Intense coordination of Eu(III) by chiral P(III) may further induce a chirality swapping from Λ- to Δ-Eu(III) (or Δ- to Λ-) forms due to stereochemical interconversion owing to the dynamic coordination ability of the ligands, as exemplified in several Eu(III) complexes and Co(III)(oxalate)3.7 The dynamic behaviour between Δ- and Λ- forms of Eu(III)(hfa)3 in fluidic solution should affect the coordination capability of four lone pairs (from two oxygen atoms) of BINAPO, as illustrated in Fig. 1.
Therefore, a significant difference in the coordination capability between acetone (two lone pairs of one oxygen) and CHCl3 (no lone pair of oxygen) is assumed to be a critical factor to determine the couplet-like and non-couplet CPL signals (Fig. 2). Although these solvents are commonly a low viscosity, acetone are usually used in preparation and spectroscopic measurements.
To verify this hypothesis, we measured 1H-NMR spectra of Eu(III)(hfa)3 with (R)-BINAP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CDCl3 and acetone-d6 (Fig. S8 and S9 in ESI†). For comparison, we obtained 1H-NMR spectra of Eu(III)(hfa)3 with (R)-BINAPO (1
1) in CDCl3 and acetone-d6 (Fig. S8 and S9 in ESI†). For comparison, we obtained 1H-NMR spectra of Eu(III)(hfa)3 with (R)-BINAPO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CDCl3 and acetone-d6, (Fig. S10 and S11 in ESI†).
1) in CDCl3 and acetone-d6, (Fig. S10 and S11 in ESI†).
Evidently, thirty two protons of (R)-BINAP (four phenyl and two naphthyl rings) showed very complex multiplet signals in the deutrated solvents (Fig. S8 and S9†). Similarly, thirty two protons of (R)-BINAPO (four phenyl and two naphthyl rings) had complex signals in these solvents (Fig. S10 and S11†). However, we were aware of the two facts as follows.
Firstly, all the proton signals of (R)-BINAP in CDCl3 showed downfield shifts of 0.03–0.06 ppm compared to those in acetone-d6. Conversely, the corresponding proton signals of (R)-BINAPO in CDCl3 showed upfield shift of ≈0.2 ppm. The coordination ability to Eu(III)(hfa)3 by the solvent molecules oppositely affected the chemical shifts of the ring protons when (R)-BINAP and (R)-BINAPO were employed as ligands. The ring protons of (R)-BINAP closer to Eu(III) efficiently feel the paramagnetic force of Eu(III) because the degree of chemical shift obeys the 1/r3 rule (r: distance between proton and Eu(III)).8
Secondary, regardless of (R)-BINAP and (R)-BINAPO, linewidths of all these proton signals in CDCl3 significantly broaden while those in acetone-d6 are very narrow. The ligand molecules at Eu(III) are assumed to be in a restricted twisting motion along C2 axis in CDCl3, whilst those at Eu(III) freely twist and fluctuate along C2 axis in acetone-d6.
Supporting from the above 1H-NMR studies, the proposed scenario should lead to an almost CPL spectroscopically single enantiomer of chiral Eu with (R)-/(S)-BINAP and Eu with (R)-/(S)-BINAPO in non-coordinating CDCl3 solution. The shapes of the CPL/PL spectra of R-Eu and S-Eu were nearly identical to those of several chiral Eu(III) complexes isolated chromato-graphically.5,9
On the other hand, in acetone, P(III) of (R)-BINAP and O of (R)-BINAPO equally coordinate to Δ- and Λ-forms of Eu(III)(hfa)3. The resulting complexes are diastereomeric pairs of Δ-Eu–(R)-BINAP and Λ-Eu–(R)-BINAP and Δ-Eu–(R)-BINAPO and Λ-Eu–(R)-BINAPO, which led to the couplet-like CPL signal at the 5D0 → 7F2 band.
Additionally, the 31P-NMR spectrum of BINAP in the presence of Eu(III)(hfa)3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CDCl3 revealed a signal at δ −15.052 ppm, which was shifted slightly upfield as compared to that of P(III) of BINAP (δ −14.862 ppm, typical for P(III), Fig. S12 and S13 in ESI†). This weak chemical shift arises from the dynamic coordination of Eu(III) by P(III) in non-coordinating CDCl3. The dynamic behaviour of coordinating ligands with Eu(III) has been reported in a recent review.7a Notably, the 31P NMR spectrum of P(V) of BINAPO in the presence of Eu(III) in CDCl3 showed a singlet at δ −69.167 ppm, which was shifted upfield from that of BINAPO (δ 28.912 ppm). This is typical for P(V), due to the strong coordinating ability of O in BINAPO (ESI, Fig. S14 and S15†).
1) in CDCl3 revealed a signal at δ −15.052 ppm, which was shifted slightly upfield as compared to that of P(III) of BINAP (δ −14.862 ppm, typical for P(III), Fig. S12 and S13 in ESI†). This weak chemical shift arises from the dynamic coordination of Eu(III) by P(III) in non-coordinating CDCl3. The dynamic behaviour of coordinating ligands with Eu(III) has been reported in a recent review.7a Notably, the 31P NMR spectrum of P(V) of BINAPO in the presence of Eu(III) in CDCl3 showed a singlet at δ −69.167 ppm, which was shifted upfield from that of BINAPO (δ 28.912 ppm). This is typical for P(V), due to the strong coordinating ability of O in BINAPO (ESI, Fig. S14 and S15†).
Comparison of the 19F-NMR spectra of hfa, Eu(hfa)3, (R)-BINAP with Eu(hfa)3 (=1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (R)-BINAPO with Eu(hfa)3 (=1
1) and (R)-BINAPO with Eu(hfa)3 (=1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in CDCl3 supported the above idea: (R)-BINAP-Eu(hfa)3 exhibited a signal at −79.099 ppm, (R)-BINAPO-Eu(hfa)3 at −78.549 ppm, Eu(hfa)3 at −79.059 ppm, and hfa at −76.500 ppm (Fig. S16–S19 in ESI†).
1) in CDCl3 supported the above idea: (R)-BINAP-Eu(hfa)3 exhibited a signal at −79.099 ppm, (R)-BINAPO-Eu(hfa)3 at −78.549 ppm, Eu(hfa)3 at −79.059 ppm, and hfa at −76.500 ppm (Fig. S16–S19 in ESI†).
The ΦPL value of R-Eu in chloroform, which originated mainly from the 5D0 → 7F2 band, was 10%. The ΦPL value decreased by one-third as compared to that of BINAPO–Eu(III) in CHCl3. The lower ΦPL was attributed to the mismatched energy level of the BINAP triplet state and Eu(III) 5D0 state and/or a rotational freedom of multiple P–C(Ar) single bonds, due to the deceased steric hindrance of P(III) of BINAP.9,10
The CD and UV-vis spectra of R-Eu (blue lines) and S-Eu (red lines) in CHCl3 and acetone are shown in Fig. 5. The characteristic π–π* UV bands of the naphthyl groups in (R)-BINAP were observed between 280 and 360 nm. The mirror-image CD spectra of R-Eu and S-Eu in CHCl3 indicate that they are an enantiomeric pair in the ground state. The |gabs| value at the first Cotton CD band (λCD = 336 nm) due to the BINAP moiety was 0.58 × 10−3. Although the λCD of BINAP of (R)-BINAP with Eu(III) in CHCl3 was the same as that of (R)-BINAP in CHCl3 (λCD = 335 nm), the |gabs| value was slightly smaller than that of (R)-BINAP with |gabs| ≈ 2.3 × 10−3 due to the coordination of Eu(III) by (R)-BINAP.10 These CD results showed that the intense coordinating ability of P(III) with Eu(III) in CHCl3 as well as acetone.
Conclusions
The first comparative study of the spectroscopic properties between BINAP and BINAPO with Eu(III)(hfa)3 in EtOH-free CHCl3 and acetone was carried out. BINAP–Eu(III) and BINAPO–Eu(III) luminophores were prepared by mixing the ligands with Eu(III)(hfa)3 in CHCl3 and acetone. The soft base P(III) of BINAP as well as the hard base O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(V) of BINAPO resulted in CPL-active Eu(III) luminophores. These results provide significant insight into how chiral complexes comprised of Eu(III) and P(III) with moderately high gem values and a high ΦPL value can be designed. Various chiral arylated P(III) ligands should be applicable to design Eu(III), possibly other lanthanoid(III). Luminophores based on chiral P(III) ligands with CPL characteristics in the visible and near infrared region will be designed in the near future.11
P(V) of BINAPO resulted in CPL-active Eu(III) luminophores. These results provide significant insight into how chiral complexes comprised of Eu(III) and P(III) with moderately high gem values and a high ΦPL value can be designed. Various chiral arylated P(III) ligands should be applicable to design Eu(III), possibly other lanthanoid(III). Luminophores based on chiral P(III) ligands with CPL characteristics in the visible and near infrared region will be designed in the near future.11
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (No. 15K05489) from MEXT/JSPS and MEXT- Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018.Notes and references
- (a) M. D. Fryzuk, T. S. Haddad and D. J. Berg, Coord. Chem. Rev., 1990, 99, 137–212 CrossRef CAS; (b) M. D. Fryzuk, T. S. Haddad, D. J. Berg and S. J. Rettig, Pure Appl. Chem., 1991, 63, 845–850 CrossRef CAS.
- (a) K. V. Katti and R. G. Cavell, Organometallics, 1989, 8, 2147–2153 CrossRef CAS; (b) D. Ferraris, B. Young, T. Dudding and T. Lectka, J. Am. Chem. Soc., 1998, 120, 4548–4549 CrossRef CAS.
- (a) R. G. Pearson, J. Chem. Educ., 1968, 45, 581–587 CrossRef CAS; (b) R. G. Pearson, J. Chem. Educ., 1968, 45, 643–648 CrossRef CAS; (c) D. Datta, Inorg. Chem., 1992, 31, 2797–2801 CrossRef CAS.
- (a) Y. Hasegawa, Y. Kimura, K. Murakoshi, Y. Wada, J.-H. Kim, N. Nakashima, T. Yamanaka and S. Yanagida, J. Phys. Chem., 1996, 100, 10201–10205 CrossRef CAS; (b) K. Nakamura, Y. Hasegawa, H. Kawai, N. Yasuda, N. Kanehisa, Y. Kai, T. Nagamura, S. Yanagida and Y. Wada, J. Phys. Chem. A, 2007, 111, 3029–3037 CrossRef CAS PubMed; (c) K. Nakamura, Y. Hasegawa, H. Kawai, N. Yasuda, Y. Tsukahara and Y. Wada, Thin Solid Films, 2008, 516, 2376–2381 CrossRef CAS; (d) Y. Hasegawa, R. Hieda, T. Nakagawa and T. Kawai, Helv. Chim. Acta, 2009, 92, 2238–2248 CrossRef CAS; (e) K. Miyata, Y. Hasegawa, Y. Kuramochi, T. Nakagawa, T. Yokoo and T. Kawai, Eur. J. Inorg. Chem., 2009, 4777–4785 CrossRef CAS.
- (a) T. Harada, Y. Nakano, M. Fujiki, M. Naito, T. Kawai and Y. Hasegawa, Inorg. Chem., 2009, 48, 11242–11250 CrossRef CAS PubMed; (b) T. Harada, Y. Hasegawa, Y. Nakano, M. Fujiki, M. Naito, T. Wada, Y. Inoue and T. Kawai, J. Alloys Compd., 2009, 488, 599–602 CrossRef CAS; (c) H. Tsumatori, T. Harada, J. Yuasa, Y. Hasegawa and T. Kawai, Appl. Phys. Express, 2011, 4, 011601–011603 CrossRef; (d) Y. Kuramochi, T. Nakagawa, T. Yokoo, J. Yuasa, T. Kawai and Y. Hasegawa, Dalton Trans., 2012, 41, 6634–6640 RSC; (e) T. Harada, H. Tsumatori, K. Nishiyama, J. Yuasa, Y. Hasegawa and T. Kawai, Inorg. Chem., 2012, 18, 6476–6485 CrossRef PubMed; (f) J. Yuasa, H. Ueno and T. Kawai, Chem.–Eur. J., 2014, 20, 8621–8627 CrossRef CAS PubMed.
- T.-Y. Li, Y.-M. Jing, X. Liu, Y. Zhao, L. Shi, Z. Tang, Y.-X. Zheng and J.-L. Zuo, Sci. Rep., 2015, 5, 14912 CrossRef CAS PubMed.
- (a) H. Miyake, Symmetry, 2014, 6, 880–895 CrossRef; (b) K. L. Stevenson and J. F. Verdieck, J. Am. Chem. Soc., 1968, 90, 2974–2975 CrossRef CAS.
- (a) C. C. Hinckley, J. Am. Chem. Soc., 1969, 91, 5160–5162 CrossRef CAS PubMed; (b) R. E. Rondeau and R. E. Sievers, J. Am. Chem. Soc., 1971, 93, 1522–1524 CrossRef.
- (a) S. D. Bonsall, M. Houcheime, D. A. Straus and G. Muller, Chem. Commun., 2007, 3676–3678 RSC; (b) J. Yuasa, T. Ohno, K. Miyata, H. Tsumatori, Y. Hasegawa and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9892–9902 CrossRef CAS PubMed; (c) F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS PubMed.
- Y. Kono, K. Nakabayashi, S. Kitamura, R. Kuroda, M. Fujiki and Y. Imai, Tetrahedron, 2015, 71, 3985–3989 CrossRef CAS.
- For recent reviews, see: (a) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem.–Eur. J., 2015, 21, 13488–13500 CrossRef PubMed; (b) J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed; (c) F. Zinna and L. Di Bari, Chirality, 2015, 27, 1–13 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the CD, CPL, NMR, and MASS spectra. See DOI: 10.1039/c6ra05856f |
| This journal is © The Royal Society of Chemistry 2016 |