Diselenide-based probe for the selective imaging of hypochlorite in living cancer cells†
Youngsam
Kim
ab,
Minsuk
Choi
c,
Sudesh T.
Manjare
d,
Sangyong
Jon
c and
David G.
Churchill
*ab
aMolecular Logic Gate Laboratory, Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea. E-mail: dchurchill@kaist.ac.kr
bCenter for Catalytic Hydrocarbon Functionalizations, Institute for Basic Science (IBS), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
cDepartment of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
dDepartment of Chemistry, University of Mumbai, Vidyanagari, Santacruz (E), Mumbai 400098, India
First published on 24th March 2016
Abstract
A non-traditional and robust probe skeleton was derivatized for chemosensing applications to investigate a potential novel mode of hypochlorite detection. The BDPP-DSe probe gave a ∼180-fold “turn-on” response to hypochlorite. Confocal fluorescence imaging demonstrated detection of hypochlorite in living cells and cell membrane permeability.
Reactive oxygen species (ROS) are generated during aerobic metabolism. ROS play an important role in many biological systems such as apoptosis, gene expression and activation of cell signalling cascades.1 When cells produce ROS beyond its antioxidant capacity, damage to lipids, proteins, and DNA may occur.2 It is a major cause of cellular damage and cell death resulting in many disorders, e.g. cancers, neurodegenerative diseases and cardiovascular diseases.3 Hypochlorous acid (HOCl), an important ROS, is produced from H2O2 with chloride ion (Cl−), via myeloperoxidase (MPO) – catalyzed oxidation. HOCl is a main immune inorganic compound that has an effect on a broad range of microorganisms during the antibacterial process.4 However, an overproduction of HOCl can cause tissue damage; the active scavenging of excess HOCl reverses injurious effects. HOCl also naturally reacts with diverse oxidizable biomolecules such as thiols, nitrogen-based compounds, and unsaturated carbon–carbon bonds.5 This oxidation leads to some diseases e.g., tubulointerstitial disease,6 cystic fibrosis,7 arthritis,8 atherosclerosis, inflammatory diseases and cancers.9 Because of their enormous biological importance, selective sensing of HOCl has received enormous and continued attention in the research literature.10
The derivatives of pyrazole have received continued attention due to reports of diverse pharmacological activities: antimicrobial, anticancer, ACE inhibitory, antiviral, and anti-inflammatory.11 Pyrazolopyridine has also demonstrated biological activity against e.g., bacteria,12 microbes,13 and leishmania.14 Selenium (Se) is an essential trace element in human biology. Compounds of Se are known as antioxidants of radical species. There is evidence that selenoproteins influence antioxidant defence.15 Se compounds are also effective in alleviating several types of cancer, cardiovascular and neurodegenerative diseases according to animal and human research data.16 These properties of selenium compounds have led researchers to synthesize selenium-based sensors for the detection of ROS.17
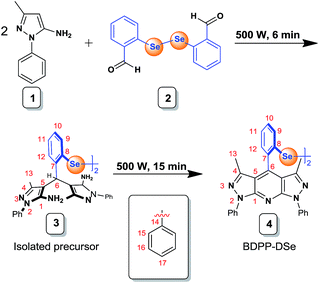
In this work, we have introduced a system for ROS detection that involves a novel dipyrazolopyridine (DPP) based-probe bearing a bridging diselenide group. Importantly, DPP derivatives have been utilized usually as a material for blue light-emitting diodes;18 these derivatives have, as of yet, not been studied in the chemosensing field to the best of our knowledge. This design involving two linked fluorophores involves two oxidizable electron-rich Se centers. This strategy was intended to promote both high sensitivity and selectivity. We began with the synthesis of 1,2-bis(2-(3,5-dimethyl-1,7-diphenyl-1,7-dihydrodipyrazolo[3,4-b:4′,3′-e]pyridin-4-yl)phenyl)diselane (BDPP-DSe) using microwave irradiation to 5-amino-3-methyl-1-phenylpyrazole (1) with bis(ortho-formylphenyl)diselenide (2) under solvent-free conditions.19 Bis(ortho-formylphenyl)diselenide (2) was prepared by ortho-chlorobenzaldehyde with sodium diselenide.20 Compound (3), the direct precursor to BDPP-DSe (4), was generated as an intermediate. Probe (4) was obtained by irradiation of compound (3) for 15 min (Scheme 1).
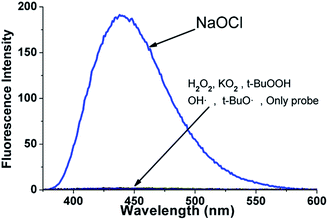
Based on the presence of the diselenide group, probe 4 was tested with various ROS such as KO2, H2O2, NaOCl, tBuOOH, OH˙, tBuO˙ (1.0 × 10−4 M) in acetonitrile (Fig. 1) and acetonitrile-pH 7.4 PBS buffer solution (Fig. S13†), where BDPP-DSe was maintained at 1.0 × 10−5 M. The absorption and emission spectral λmax values were 331 and 436 nm, respectively. BDPP-DSe showed a rapid and strong blue emission response for NaOCl. A ∼180-fold intensity increase was observed. On the other hand, the other ROS (KO2, H2O2, tBuOOH, OH˙, tBuO˙) did not impart any fluorescence change to BDPP-DSe. To confirm that competition and interference exists from other reactive oxygen species was negligible, NaOCl (1.0 × 10−4 M) was added to the solution containing BDPP-DSe and other ROS (KO2, H2O2, tBuOOH, OH˙, tBuO˙; 10 × 10−4 M). Even though the fluorescence enhancement was slightly less than that found for the one treated with NaOCl only, these results suggested that the presence of the other ROS does not affect the activity of NaOCl (Fig. S14†).
The fast enhancement of probe fluorescence with NaOCl encouraged us to determine the response time. The time-course response of the BDPP-DSe probe (1.0 × 10−5 M) with NaOCl (1.0 × 10−4 M) was tested. The emission intensity reflected an equilibrium state within ∼8 min (Fig. S15†). We also observed that the emission intensity of the chemically oxidized probe was retained. The fluorescence of the “switched-on” probe in the presence of excess oxidant was not quenched even at 15 days (rt) under white light (Fig. S16†). These results support that dipyrazolopyridine-based fluorescence in solution is exceptionally stable, as predicted from its extensive employment in OLED applications; also the reaction that leads to enhanced fluorescence is rapid. Considering the application to the damaged tissues which have locally low pH, the robust BDPP-DSe was tested to confirm the selective detection of hypochlorite over other ROS in solution (1.0 × 10−5 M, acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, v/v, 8
water, v/v, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
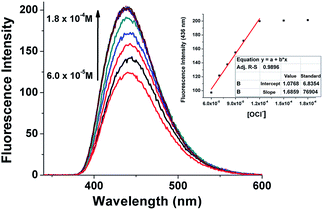
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, pH 1.1–4.6) (Fig. S17†).21 The result indicated that BDPP-DSe also could be used in acidic conditions such as in cancer cells and in damaged tissues. The sensitivity of BDPP-DSe was tested with various concentrations of NaOCl (6.0 × 10−5 M to 1.8 × 10−4 M) in the presence of BDPP-DSe (1.0 × 10−5 M). The emission intensity was saturated upon the addition of 1.2 × 10−4 M of NaOCl (12 equiv.), and was found to be linearly proportional to the concentration of NaOCl. The detection limit was calculated to be 3.6 × 10−7 M (Fig. 2). Considering the possibility of the presence of damaging concentrations of HOCl to mammalian cells exist below 2.5 × 10−5 M, BDPP-DSe is potentially used to detect abnormal concentrations of HOCl in cells.22
2, pH 1.1–4.6) (Fig. S17†).21 The result indicated that BDPP-DSe also could be used in acidic conditions such as in cancer cells and in damaged tissues. The sensitivity of BDPP-DSe was tested with various concentrations of NaOCl (6.0 × 10−5 M to 1.8 × 10−4 M) in the presence of BDPP-DSe (1.0 × 10−5 M). The emission intensity was saturated upon the addition of 1.2 × 10−4 M of NaOCl (12 equiv.), and was found to be linearly proportional to the concentration of NaOCl. The detection limit was calculated to be 3.6 × 10−7 M (Fig. 2). Considering the possibility of the presence of damaging concentrations of HOCl to mammalian cells exist below 2.5 × 10−5 M, BDPP-DSe is potentially used to detect abnormal concentrations of HOCl in cells.22
In this system, the diselenide is the most readily oxidizable part of BDPP-DSe. BDPP-DSe was treated with excess NaOCl to demonstrate the oxidation of the [–Se–Se–] group. The oxidation of diselenide was confirmed by 1H and 77Se NMR spectroscopy and mass spectrometry (Fig. S18–S21†). The 77Se NMR singlet peak assigned to oxidized form of BDPP-DSe (BDPP-DSeO) was found to be downfield-shifted at δ 1018.1 ppm (Fig. S18†), compared with the 77Se NMR peak of BDPP-DSe found at 405.0 ppm. The 1H NMR signals of diphenyl protons were also affected markedly (Fig. S19 and 20†).
Excess NaOCl (20 μL from 4% solution) was added to a sample of the probe (4 mg, 0.4 mmol) in acetonitrile solvent. After 30 minutes at room temperature, the sample was submitted for mass spectral analysis. The mass spectrum shows signals consistent with species having been affected by chemical oxidation. While, the starting material is shown in the MS experiment ([BDPP-DSe + Na]+) bearing an isotopic pattern consistent with the presence of two selenium atoms, a product of oxidation (4 oxygen atoms) with the same isotopic pattern is shown ([BDPP-DSeO2 + Na]+ – calc.:1075.1457, observed: 1075.1484). An accompanying peak corresponding to a monomeric form ([DPP-SeHO2 + Na]+), currently assigned to the seleninic acid is also present at a m/z of (calc.: 550.0753, obtained: 550.0674) with much higher intensity. It is thought that since the Se–Se is initially present, as seen with an analogous system the selenenic acid formation is a result of the excess oxidation imparted to the electron rich Se–Se group. Se–Se bond scission thus ensues. Due to the framework at hand, it is currently thought that the important oxidation that affects the turn-on fluorescence is due only to the selenide oxidation.17e Rupture of the Se–Se bond, apparent in the mass spectrum, is consistent with the difficulty in effecting facile reversibility of this probe (reformation of the reduced Se–Se group) in this reaction under the use of the current chemical oxidant. We believe that the major form of BDPP-DSeO may be a DPP monomer with a phenylseleninic acid functionality. From these results, we attributed the ‘turn-on’ response to the formation of BDPP-DSeO. With this chemical modification, the quenching of the photoinduced electron transfer (PET) mechanism that existed between a phenyl selenide group and a BDPP is lifted, thus allowing for dipyrazolopyridine-based fluorescence. Previously, the PET quenching-type mechanism involving a related selenide-containing probe compound was discussed in detail.17
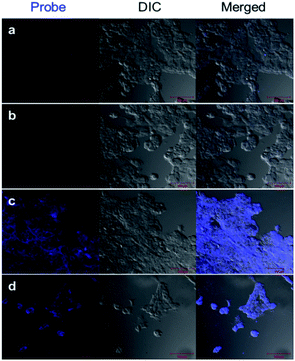
Finally, we performed confocal fluorescence imaging for testing BDPP-DSe with human breast cancer cells (MCF-7) to help visualize HOCl concentrations in such living cells. For cell internalization tests, MCF-7 cells were seeded on 18 mm ø cover-slips into 12-well plates and cultured overnight. MCF-7 cells were treated with BDPP-DSe (1.0 × 10−5 M in D-PBS) for 30 min (Fig. 3b); the cells were then incubated with NaOCl (1.0 × 10−4 M) for 15 min (Fig. 3c). As expected, a bright blue fluorescence signal was observed throughout the cytoplasm of the MCF-7 cells. These results suggested that BDPP-DSe possesses good cell membrane permeability generally in detecting HOCl in living cells. We also examined whether BDPP-DSe could detect HOCl from H2O2-induced ROS production in MCF-7 cells. Hydrogen peroxide (H2O2) was used for the production of ROS. As shown in Fig. 3d, MCF-7 cells were incubated with 0.1% H2O2 in cell culture media for 1 h and then treated with BDPP-DSe (1.0 × 10−5 M) for 30 min. Blue fluorescence was also observed throughout the cytoplasm of the MCF-7 cells. These results support that BDPP-DSe can detect −OCl in the cytoplasm of the cells and is able to help visualize HOCl from the H2O2-induced ROS production in cancer cells (Fig. 3).
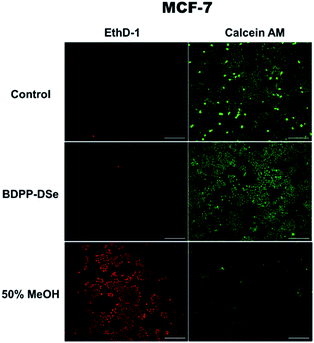
In order to demonstrate the toxicity of BDPP-DSe, cell viability testing was performed. The viability of cells cultured on cover glass was determined using a LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies, Foster City). Approximately 2 × 105 cells were grown on cover-glass (18 mm ø) in a 12-well plate for 24 h at 37 °C. Cell assays included both an untreated control group and a group that was treated with BDPP-DSe (1.0 × 10−5 M), or 50% methanol (MeOH), for 30 minutes. After washing with D-PBS, cells were then treated with 2 μM calcein AM and 4 μM EthD-1 for 30 minutes at room temperature. Cells were then fixed with 4% paraformaldehyde and examined under a fluorescence microscope (Eclipse 80i, Nikon) to determine the presence of live and dead cells (Fig. 4). Also, a few reported examples for LC50 of pyrazole moieties were previously investigated to help support that pyrazole moieties have relatively low toxicity.23
Conclusions
We report a convenient synthesis of a novel diselenide-based dipyrazolopyridine probe through the use of a domestic microwave oven; we have fully characterized the probe and its precursor spectroscopically. As per our knowledge, BDPP-DSe is the first probe using the DPP system in chemosensing. The skeleton has been used extensively in OLED research. However, the pyrazolo-free-N position can later be used for placement of a methyl group and in helping establish particular hydrogen bonding interactions. Probe, BDPP-DSe, shows a selective “turn-on” response resulting in a NaOCl ∼180-fold fluorescence intensity increase. Competitive testing with H2O2, KO2, tBuOOH, OH˙, tBuO˙ do not result in optical changes and also support the selectivity of BDPP-DSe in probing NaOCl. Confocal microscopy imaging was carried out in living breast cancer cells. These fluorescence imaging studies demonstrated detection of hypochlorite in living cells and cell membrane permeability. From these results, we believe that BDPP-DSe is potentially useful in various fields such as neuroscience and health science as well as in specific diseases such as brain-related diseases and cancer.Acknowledgements
The Molecular Logic Gate Laboratory operated by Prof. D. G. Churchill acknowledges support from (i) Mid-Career Researcher Program through the NRF (National Research Foundation) of Korea (NRF-2014R1A2A1A11052980) funded by MEST and (ii) Institute for Basic Science (IBS) for financial support. Mr Youngsam Kim acknowledge IBS research Fellowships and KAIST for providing research facilities.Notes and references
- J. T. Hancock, R. Desikan and S. J. Neill, Biochem. Soc. Trans., 2001, 29, 345–350 CrossRef CAS PubMed.
- V. J. Thannickal and B. L. Fanburg, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2000, 279, L1005–L1028 CAS.
- (a) G. Waris and H. Ahsan, J. Carcinog., 2006, 5, 14 CrossRef PubMed; (b) B. Uttara, A. V. Singh, P. Zamboni and R. T. Mahajan, Curr. Neuropharmacol., 2009, 7, 65–74 CrossRef CAS PubMed.
- (a) C. C. Winterbourn, M. C. Vissers and A. J. Kettle, Curr. Opin. Hematol., 2000, 7, 53–58 CrossRef CAS PubMed; (b) L. Wang, M. Bassiri, R. Najafi, K. Najafi, J. Yang, B. Khosrovi, W. Hwong, E. Barati, B. Belisle, C. Celeri and M. C. Robson, J. Burns Wounds, 2007, 6, 65–79 Search PubMed; (c) Z. M. Prokopowicz, F. Arce, R. Biedron, C. L. Chiang, M. Ciszek, D. R. Katz, M. Nowakowska, S. Zapotoczny, J. Marcinkiewicz and B. M. Chain, J. Immunol., 2010, 184, 824–835 CrossRef CAS PubMed.
- E. Malle, T. Buch and H.-J. Grone, Kidney Int., 2003, 64, 1956–1967 CrossRef CAS PubMed.
- H. Scheuer, W. Gwinner, J. Hohbach, E. F. Grone, R. P. Brandes, E. Malle, C. J. Olbricht, A. K. Walli and H.-J. Grone, Am. J. Physiol.: Renal, Fluid Electrolyte Physiol., 2000, 278, F63–F74 CAS.
- K. Akong-Moore, O. A. Chow, M. von Kockritz-Blickwede and V. Nizet, PLoS One, 2012, 7, e42984 CAS.
- L. K. Stamp, I. Khalilova, J. M. Tarr, R. Senthilmohan, R. Turner, R. C. Haigh, P. G. Winyard and A. J. Kettle, Rheumatology, 2012, 51, 1796–1803 CrossRef CAS PubMed.
- D. I. Pattison and M. J. Davies, Chem. Res. Toxicol., 2001, 14, 1453–1464 CrossRef CAS PubMed.
- (a) Q. Xu, K. A. Lee, S. Lee, K. M. Lee, W. J. Lee and J. Yoon, J. Am. Chem. Soc., 2013, 135, 9944–9949 CrossRef CAS PubMed; (b) S.-R. Liu and S.-P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS PubMed; (c) S. T. Manjare, J. Kim, Y. Lee and D. G. Churchill, Org. Lett., 2014, 16, 520–523 CrossRef CAS PubMed; (d) A. M. Palazzolo, C. Suquet, M. E. Konkel and J. K. Hurst, Biochemistry, 2005, 44, 6910–6919 CrossRef CAS PubMed; (e) Y. W. Yap, M. Whiteman, B. H. Bay, Y. Li, F.-S. Sheu, R. Z. Qi, C. H. Tan and N. S. Cheung, J. Neurochem., 2006, 98, 1597–1609 CrossRef CAS PubMed; (f) Y. Zhou, J. Y. Li, K. H. Chu, K. Liu, C. Yao and J. Y. Li, Chem. Commun., 2012, 48, 4677–4679 RSC.
- A. Chauhan, P. K. Sharma and N. Kaushik, Int. J. ChemTech Res., 2011, 3, 11–17 Search PubMed.
- N. Panda, S. Karmakar and A. K. Jena, Chem. Heterocycl. Compd., 2011, 46, 1500–1508 CrossRef CAS.
- S. Abu-Melha, Arch. Pharm., 2013, 346, 912–921 CrossRef CAS PubMed.
- H. D. Mello, A. Echevarria, A. M. Bernardino, M. Canto-Cavalheiro and L. Leon, J. Med. Chem., 2004, 47, 5427–5432 CrossRef PubMed.
- (a) G. Mugesh, W. W. du Mont and H. Sies, Chem. Rev., 2001, 101, 2125–2179 CrossRef CAS PubMed; (b) A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357–4416 CrossRef CAS PubMed.
- R. R. Ramoutar and J. L. Brumaghim, Cell Biochem. Biophys., 2010, 58, 1–23 CrossRef CAS PubMed.
- (a) G. Li, D. Zhu, Q. Liu, L. Xue and H. Jiang, Org. Lett., 2013, 15, 2002–2005 CrossRef CAS PubMed; (b) S.-R. Liu and S.-P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS PubMed; (c) B. Wang, P. Li, F. Yu, J. Chen, Z. Qu and K. Han, Chem. Commun., 2013, 49, 5790–5792 RSC; (d) B. Wang, P. Li, F. Yu, P. Song, X. Sun, S. Yang, Z. Lou and K. Han, Chem. Commun., 2013, 49, 1014–1016 RSC; (e) S. T. Manjare, S. Kim, W. D. Heo and D. G. Churchill, Org. Lett., 2014, 16, 410–413 CrossRef CAS PubMed; (f) Y. Kim, S. V. Mulay, M. Choi, S. B. Yu, S. Jon and D. G. Churchill, Chem. Sci., 2015, 6, 5435–5439 RSC; (g) S. T. Manjare, Y. Kim and D. G. Churchill, Acc. Chem. Res., 2014, 47, 2985–2998 CrossRef CAS PubMed; (h) L. Lou, P. Li and K. Han, Acc. Chem. Res., 2015, 48, 1358–1368 CrossRef PubMed.
- (a) E. Balasubramaniam, Y. T. Tao, A. Danel and P. Tomasik, Chem. Mater., 2000, 12, 2788–2793 CrossRef CAS; (b) Y. T. Tao, C. H. Chuen, C. W. Ko and J. W. Peng, Chem. Mater., 2005, 14, 4256–4261 CrossRef.
- J. Quiroga, J. Portilla, B. Insuasty and R. Abonía, J. Heterocycl. Chem., 2005, 42, 61–66 CrossRef CAS.
- S. Panda, S. S. Zade, H. B. Singh and G. Wolmershäuser, J. Organomet. Chem., 2005, 690, 3142–3148 CrossRef CAS.
- (a) J. R. Griffiths, Br. J. Cancer, 1991, 64, 425–427 CrossRef CAS PubMed; (b) I. F. Tannock and D. Rotin, Cancer Res., 1989, 49, 4373–4384 CAS.
- (a) N. S. Gould, S. Gauthier, C. T. Kariya, E. Min, J. Huang and D. J. Brian, Respir. Res., 2010, 11, 119–128 CrossRef PubMed; (b) N. Gungor, A. M. Knaapen, A. Munnia, M. Peluso, G. R. Haenen, R. K. Chiu, R. W. Godschalk and F. J. van Schooten, Mutagenesis, 2010, 25, 149–154 CrossRef PubMed.
- (a) F. M. Abdelrazek, N. H. Metwally and N. A. Sobhy, AFINIDAD LXV 2008, 538, 482–487; (b) S. A. F. Rostom, M. A. Shalaby and M. A. El-Demellawy, Eur. J. Med. Chem., 2003, 38, 959–974 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 77Se and 2D NMR, HRMS spectra of compounds, details of the synthetic procedure, additional fluorescent spectra. See DOI: 10.1039/c6ra04257k |
| This journal is © The Royal Society of Chemistry 2016 |