Motion in microfluidic ratchets
D.
Caballero
abc,
J.
Katuri
ad,
J.
Samitier
abc and
S.
Sánchez
*ade
aInstitute for Bioengineering of Catalonia (IBEC), Baldiri Reixac 15-21, 08028 Barcelona, Spain. E-mail: ssanchez@ibecbarcelona.eu
bCentro de Investigación Biomédica en Red en Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Madrid, Spain
cDepartment of Engineering: Electronics, University of Barcelona, 08028 Barcelona, Spain
dMax Planck Institute for Intelligent Systems, Heisenbergstrasse 3, Stuttgart, Germany. E-mail: ssanchez@is.mpg.de
eCatalan Institute for Research and Advanced Studies (ICREA), Psg. Lluís Companys, 23, 08010 Barcelona, Spain
First published on 19th October 2016
Abstract
The ubiquitous random motion of mesoscopic active particles, such as cells, can be “rectified” or directed by embedding the particles in systems containing local and periodic asymmetric cues. Incorporated on lab-on-a-chip devices, these microratchet-like structures can be used to self-propel fluids, transport particles, and direct cell motion in the absence of external power sources. In this Focus article we discuss recent advances in the use of ratchet-like geometries in microfluidics which could open new avenues in biomedicine for applications in diagnosis, cancer biology, and bioengineering.
Introduction
Periodic, asymmetrically-structured surfaces can be used to obtain unidirectional motion of driven or self-propelled objects. Physicists define these asymmetric features which ‘rectify’ the random motion of out-of-equilibrium particles into directional motion as ratchets. This concept has been studied extensively in several fields, particularly in biology, where multiple biological phenomena were shown to depend on this mechanism, such as the directed motion of molecular motors. Ratchets have also been used to study the motion of other types of complex active particles, such as cells. This has attracted the attention of both physicists and biologists, and a number of biophysical models describing directed cell motility based on ratchet approaches have emerged.1–3The development of microfluidic devices which incorporate ratchet-like structures within microchannels to direct the transport of fluids, particles, and cells has received significant attention as a new way to direct motion in the absence of an external power source. These new techniques provide multiple advantages compared to standard techniques, which require the use of external sources, such as bulky pumps, pressure gradients, or oscillatory electrical fields. This will allow the development of novel autonomous microfluidic devices with promising applications in biomedicine. In this Focus article, we summarize some recent works combining microfluidics with ratchet-based propulsion systems to direct fluids along microchannels, transport passive and active particles, and sort and guide cells within microfluidic devices.
Ratchet-based liquid propulsion systems
The ability to control fluid flow in microfluidic devices is of great importance in many domains. Typically, flow motion is controlled externally by imposing a pressure difference. Recently, it was demonstrated that asymmetric micro- and nano-sized features incorporated within microfluidic systems could also autonomously transport fluids and droplets unidirectionally along microchannels. This was shown by Bae et al. who combined standard UV photolithography processes with the use of a prism array to integrate in situ asymmetric sawteeth micro-structures within a microchannel.4 With this technique, the angle of the embedded microstructures – ratchets – could be precisely controlled, permitting the fabrication of ratchet structures with different orientation angles (Fig. 1a). The liquid was passively introduced inside the chip by gravity and the directional wetting of the asymmetric microstructures enabled fluid flow in a preferred direction, whereas isotropic structures caused no preferential direction of the flow. The direction of flow was determined by the difference in the critical contact angles of the ratchets, which forced the liquid to move unidirectionally in the direction of the lower angle value. The orientation angle also influenced flow rate along channels, where fluid flow was at a higher speed on aligned ratchet structures whereas it was delayed for reversed structures (Fig. 1b). This difference was a consequence of the different net forces (inlet pressure over friction) on the contacting surfaces, which depended on the base angles of the ratchet microstructures.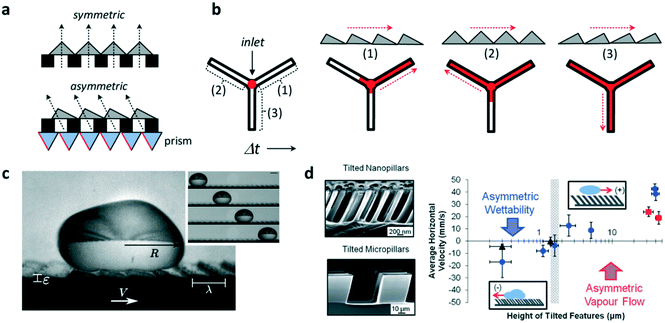 | ||
| Fig. 1 Application of ratchet-like microstructures for fluid transport. (a) Scheme showing the fabrication of polymeric isotropic and anisotropic structures for the unidirectional transport of fluids. (b) Different ratchet configurations within a microchannel induce different fluid behaviors with different fluid flow speeds. (c) A drop of water self-propels when deposited on a hot surface containing asymmetric features. (d) (Left) Scanning electron microscopy image of tilted micro- and nano-pillars. (Right) Average horizontal velocity vs. tilted pillars height showing the two size-dependent mechanisms governing the motion of dynamic Leidenfrost droplets. Reprinted from ref. 5 (c) and ref. 6 (d) with permission. Panels (a) and (b) adapted from ref. 4. | ||
Liquid droplets were also shown to self-propel if placed on ratchet-like structures. For this to occur, liquids must be in the so-called Leidenfrost state, when a drop levitates if placed on a hot surface whose temperature is far above their boiling point. The generated vapor flow sustains and propels the drop with reduced friction with the underlying substrate towards the direction set by the ratchet. However, the elevated temperatures needed to reach the Leidenfrost state limit the lifetime of the drop motion, which, in the best scenario, is a few hundreds of seconds. Remarkably, coating the surface with a hydrophobic layer reduces the temperature needed to self-propel liquid drops. In this regard, Dupeux et al. used hot (100 °C) ratchet-like milli-sized structures functionalized with a superhydrophobic coating to self-propel deionized water droplets unidirectionally (Fig. 1c).5 The motion of the levitating droplets was rectified by the asymmetric teeth and directed against the feature tilt. The propulsion was a consequence of the rectification of the vapour flow by the asymmetric ratchet structures. Liquid speed was observed to hardly depend on the ratchet temperatures, but interestingly, droplet motion was observed below 100 °C which implied that levitation was not necessary to induce motion.
Liquid directionality on Leidenfrost ratchets depends on features size and orientation. Nanometric features, such as tilted pillars, have been shown to direct droplet directionality towards the same tilting direction, whereas on micro- and millimetre scale droplets move against the feature tilt angle. In order to identify the mechanism at work explaining this switch in directionality, Agapov et al. developed a dynamic Leidenfrost ratchet consisting of tilted micro- and nano-pillars, and studied the directionality of impacting deionized water droplets on a level hot (350 °C) plate (Fig. 1d, left).6 The authors revealed an interplay between asymmetric vapour flow phenomena and asymmetric wettability which determined droplet directionality. The former dominated in the case of large feature sizes (micro and milli-scale), whereas the latter governed droplet motion at the nanometric scale (Fig. 1d, right). Intriguingly, no preferential directed motion was observed when the droplets were gently deposited on the tilted pillars surface, but moved randomly. Under this gentle deposition condition, the vapor film was too thick to allow droplet–surface interaction at the top of the nanopillars and rectify droplet motion. In this case, liquid speed was observed to linearly depend on ratchet features size. Transition between both regimes (wettability vs. vapour flow) occurred when the features size reached the microscale.
In summary, these works show that directed fluid flow can be achieved by using ratchet-like structures integrated in microfluidic systems. These findings could have important applications in lab-on-a-chip devices, including the transport and targeted delivery of reagents, on-chip immunoassays or guiding chemical reactions, where the control of the direction and timing of fluid flow is critical.
Particle transport in ratcheted systems
Contact-charge electrophoresis refers to the process in which a conductive particle is first charged by contact with an electrode surface in the presence of an electric field and then translates in that electric field by electrophoresis. If a conductive particle is placed between two electrodes generating the electric field, every time the particle touches an electrode its charge changes and it begins to migrate to the opposite electrode. In effect, it is possible to obtain a rapid oscillatory motion of the particle between the two electrodes (Fig. 2a). Drews et al. demonstrated that this oscillatory motion can be rectified to obtain directed transport of micron sized particles in a microfluidic channel.7 In order to achieve this, they fabricated a microfluidic channel containing inclined PDMS barriers at alternative positions and flanked by gallium electrodes (Fig. 2b). In a medium of a dielectric liquid (mineral oil), they suspended the conductive micro-particles (silver-coated glass spheres). Upon contact with one of the electrodes, the particles acquire a net charge corresponding to the charge of the electrode and begin to translate in the opposite direction. Upon reaching the PDMS barriers at the opposite end, the particles roll down the barrier and have a net migration down the channel due to the barrier slope. Eventually they come in contact with the opposite electrode and repeat the same process in the opposite direction. This process can result in a micron sized particle translating down the channel at speeds in the order of a few mm s−1 (Fig. 2c). The authors found this directional motion to be robust even against an opposing flow. They also designed a microfluidic system to separate particles from a fluid stream using this effect. In this system (Fig. 2d), the electrodes and the PDMS barriers are designed such that the particles can only oscillate in the desired channels. The particles enter at channel 1 and exit at channel 2, while the fluid is separated into channel 3. Finally, they showed that this effect also persists for hydrogel particles, allowing for the future transport of aqueous containers carrying dissolved solutes in microfluidic systems.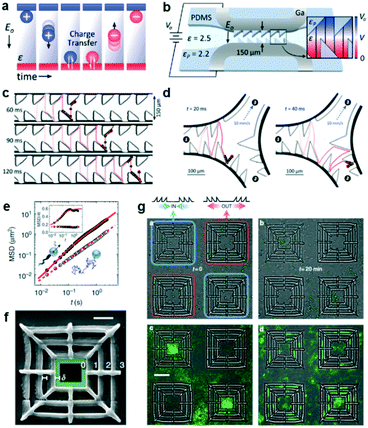 | ||
| Fig. 2 Particle transport using ratchet structures. (a) A conductive particle between two electrodes suspended in a dielectric fluid. Every time the particle makes contact with an electrode, it acquires a charge and migrates towards the opposite electrode. (b) Schematic of the ratcheted microfluidic channel used to direct particle transport. (c) Trajectory of a particle undergoing directed motion. (d) Particle trajectory in a microfluidic channel designed to separate conductive particles from fluid flow. (e) The mean squared displacements (MSD) of colloidal particles in the absence of bacteria (simple Brownian motion, open symbols) and in a bacterial bath (filled symbols). (f) A scanning electron microscopy image of a square structure that gathers particles to the centre. (g) (Upper panels) Snapshots of particles and bacteria in the initial and final state. The latter state shows the system when the particles are strongly affected by bacterial transport over asymmetric barriers. (Lower panels) Particle distributions averaged over a steady state between t1 = 15 min and t2 = 20 min (left). The right panel shows the particle distribution in an experiment without bacteria. Reprinted from ref. 7 (a–d) and ref. 8 (e–g) with permission. | ||
Another system that could spatially organize colloidal particles in an active suspension of bacteria was developed by Koumakis et al.8 Active particles, such as bacteria and self-propelled colloids, are animated by non-equilibrium stochastic forces characterized by a persistence time on the timescale of seconds. Passive colloidal particles suspended in a bath of swimming bacteria have also been shown to display random walks with a finite persistence time (Fig. 2e).9 When the persistence time is large enough to probe topological features in rigid obstacles, the particles can be rectified using effective asymmetric energy barriers. These asymmetric barriers were created by patterning a flat surface with a 3D structure containing three square boundaries with the larger slopes pointing either inwards (to collect particles) or outwards (to eject particles) (Fig. 2f and g). In the absence of bacteria, the particles sediment close to the bottom surface and are randomly distributed. The asymmetric walls present an energy barrier of 20 kBT which confines the sedimented particles. However in the presence of an active suspension of swimming bacteria, which introduces off-equilibrium fluctuating forces, the distribution of colloids is no longer homogeneous. After 20 min, the collecting structures are filled up and the ejecting structures are empty of colloids (Fig. 2g). A number of parameters, such as the height and slope of the structures or the particle size can influence the rectification process. One particular parameter that was tested was the density of the bacterial suspension. When the bacterial concentration is high, the particles acquire higher diffusivity and are less sensitive to the underlying topographical landscape making the rectification process less effective.
The two methods described above, while not exhaustive, serve as good examples of how well designed ratchet structures can be used to achieve directional transport of colloidal particles in the absence of external pressure gradients.
Cell sorting and guidance using microfluidic ratchets
In several pathologies the mechanical properties of cells are perturbed. This perturbation in cell rigidity can be used to sort and isolate pathological cells to study in vitro the evolution of the disease. Cells can be sorted by multiple means, mostly based on fluorescent and magnetic technologies. Ratchet structures provide an efficient alternative with the advantage that cells do not need to be labeled. Fig. 3a shows a microfluidic device containing an array of ratchet microstructures used to sort non-adherent cells by means of mechanical deformation. Cells are forced to pass through the asymmetric constrictions, where the gap distances between individual motifs are significantly smaller than the cell diameter. The gap gradually decreases on the different rows, permitting only the upwards transition of cells depending exclusively on their capability to deform through the blocking pore (Fig. 3b). This approach was applied by Guo et al. to isolate red blood cells (RBCs) infected with Plasmodium falciparum, which lose deformability due to the metabolism of hemoglobin by the parasite.10 A mixture of non-infected and infected RBCs was flowed in the lower region of the ratcheting array and an oscillatory upwards and downwards flow was applied (Fig. 3b, top). The former was used to force RBCs to pass through the structures while the latter was used to avoid clogging within the chip and maintain a constant filtration force, permitting cell movement mainly in one direction (Fig. 3b, lower left). This oscillatory flow, together with the difference in pressure required to deform the cells along and against the direction set by the asymmetric structures, determined the transit of cells. Finally, a lateral flow directed the deposition of cells in a reservoir for further analysis (Fig. 3b, lower right). This method provided improved selectivity over previous detection methods, being capable of detecting low parasitemia at early stages of the pathology. Finally, this cell sorting approach could also be applied to separate circulating tumor cells based on their distinct deformability relative to hematological cells.11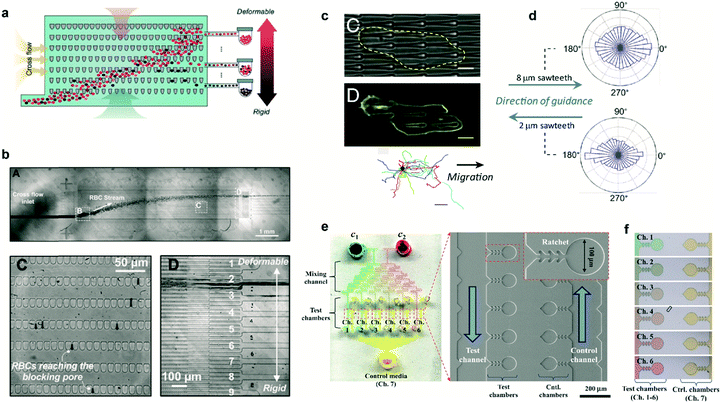 | ||
| Fig. 3 Cell sorting and guidance using ratchets. (a) Ratchet-sorting device with alternating upwards and downwards flows, and cross flow, which permits the sorting of cells depending on their mechanical deformation. (b) (Upper panel) Optical image displaying the flow of RBCs. (Lower panels) Cells are sorted depending on their membrane rigidity (left) and deposited on the appropriate outlet (right). (c) Cells seeded on a topographical ratchet bias their dynamics and direction of motion. (d) The direction of cell motion depends on the ratchet geometry and size. (e) Microbial biosensor device containing ratchet-like microchannels to direct bacteria growth. (f) Concentration-dependent experiments can be performed in bacteria-containing chambers. Reprinted from ref. 10 (a and b), ref. 14 (c and d), and ref. 15 (e and f) with permission. | ||
Ratchet-like structures can also be used to ‘rectify’ (from random to directional) the motion of motile cells in the absence of chemical or mechanical gradients.1–3,12,13 Recently, the bias of cell motility by means of ratchets was termed Ratchetaxis.1In vivo, this type of motion can synergize with chemical gradients leading to complex cell behaviors. This was addressed in vitro by Comelles et al. using a combination of a topographical ratchet coated with a fibronectin gradient.2 Depending on their respective orientations, both cues cooperated or competed in guiding cell motion. The authors identified the mechanical interaction of the cell nucleus with ratchet structures as the mechanism polarizing the cell which determined the final direction of cell motion. Note that the geometry and size of the ratchet structures can influence both the direction of motion and the organelle responsible of the bias.1,2 In this regard, Sun et al. used asymmetric sawteeth structures to bias the internal actin polymerization waves, imposing cell polarization and directing cell motility.14 The direction of cell motion depended on sawtooth length and height, suggesting the presence of multiple competing mechanisms involved in biasing actin waves (Fig. 3c). Importantly, for a specific ratchet configuration, the direction of bias was conserved for different cell types suggesting a universal intracellular mechanism responsible for breaking the symmetry (Fig. 3d). This is in contrast with former works where the direction of motion critically depended on the cell type and the selected ratchet geometry.3 This cell-dependent phenomenon provided the possibility to sort different cell types or to fabricate “cancer traps” that can be incorporated in lab-on-a-chip devices or even in vivo.3
The use of ratchet-based structures can also be extended to bacteria. In this regard, Kim et al. developed a microfluidic platform for the detection of heavy metal ions (HMIs) using microbial biosensors.15 Ratchet-shaped microchannels were embedded within the platform to self-concentrate and retain the bacteria in a microchamber array (Fig. 3e). Due to the specific geometry of the ratchet structures, swimming bacteria were forced to grow and accumulate within each individual chamber. Nutrient supply and waste disposal was ensured by constant media flow and diffusion. The developed device incorporated multiple filling channels which permitted the analysis of varying HMIs concentrations, while keeping the concentration of microbes constant in all the test chambers (Fig. 3f). In this case, microbes acted as sensing entities which were genetically modified for GFP expression. This approach improved the sensitivity for detecting Pb2+ and Cd2+ ions by approximately three orders of magnitude when compared to standard methods.15
Altogether, these works reveal that cell motion can be biased over large distances by means of ratchets. This provides multiple advantages compared to chemical gradients which degrade over time and the migrating distance is limited by the detection capability of cells, typically a few cell bodies.
Conclusions
The recent progress in microfabrication techniques and nanotechnology has allowed the production of a large plethora of ratchet-based guiding cues which, incorporated within microfluidic devices, can be used as a new transport methodology. This enables the development of autonomous microfluidic devices to self-propel fluids, guide particles, or direct cell locomotion without the need for using external pumping sources, and provides a new research direction for the design of lab-on-a-chip systems powered by ratchets. This could have important applications in biomedicine, such as transporting biological samples or guiding chemical reactions.Acknowledgements
The authors acknowledge the European Research Council under European Union 7th Framework Programme (FP7/2007-2013)/ERC Grant Agreement 311529 (Lab-in-a-tube and Nanorobotics biosensors). D. C. acknowledges the Secretary for Universities and Research of the Ministry of Economy and Knowledge of the Government of Catalonia and the COFUND program of the Marie Curie Actions of the 7th R&D Framework Program of the European Union. This work was supported by CIBER, funded by the VI National R&D&I Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions, and the Instituto de Salud Carlos III, with the support of the European Regional Development Fund. This work was also financed by the Commission for Universities and Research of the Department of Innovation, Universities, and Enterprise of the Generalitat de Catalunya (2014 SGR 1442) and by the project MINDS (TEC2015-70104-P), awarded by the Spanish Ministry of Economy and Competitiveness.Notes and references
- D. Caballero, R. Voituriez and D. Riveline, Cell Adhes. Migr., 2015, 9, 327–334 CrossRef CAS PubMed.
- J. Comelles, D. Caballero, R. Voituriez, V. Hortigüela, V. Wollrab, A. L. Godeau, J. Samitier, E. Martínez and D. Riveline, Biophys. J., 2014, 107, 1513–1522 CrossRef CAS PubMed.
- G. Mahmud, C. J. Campbell, K. J. M. Bishop, Y. A. Komarova, O. Chaga, S. Soh, S. Huda, K. Kandere-Grzybowska and B. A. Grzybowski, Nat. Phys., 2009, 5, 606–612 CrossRef CAS.
- W.-G. Bae, S. M. Kim, S.-J. Choi, S. G. Oh, H. Yoon, K. Char and K. Y. Suh, Adv. Mater., 2014, 26, 2665–2670 CrossRef CAS PubMed.
- G. Dupeux, P. Bourrianne, Q. Magdelaine, C. Clanet and D. Quéré, Sci. Rep., 2014, 4, 5280 CAS.
- R. L. Agapov, J. B. Boreyko, D. P. Briggs, B. R. Srijanto, S. T. Retterer, C. P. Collier and N. V. Lavrik, Nanoscale, 2014, 6, 9293–9299 RSC.
- A. M. Drews, H.-Y. Lee and K. J. M. Bishop, Lab Chip, 2013, 13, 4295–4298 RSC.
- N. Koumakis, A. Lepore, C. Maggi and R. Di Leonardo, Nat. Commun., 2013, 4 Search PubMed.
- X.-L. Wu and A. Libchaber, Phys. Rev. Lett., 2000, 84, 3017–3020 CrossRef CAS PubMed.
- Q. Guo, S. P. Duffy, K. Matthews, X. Deng, A. T. Santoso, E. Islamzada and H. Ma, Lab Chip, 2016, 16, 645–654 RSC.
- E. S. Park, C. Jin, Q. Guo, R. R. Ang, S. P. Duffy, K. Matthews, A. Azad, H. Abdi, T. Todenhöfer, J. Bazov, K. N. Chi, P. C. Black and H. Ma, Small, 2016, 12, 1909–1919 CrossRef CAS PubMed.
- R. Sunyer, V. Conte, J. Escribano, A. Elosegui-Artola, A. Labernadie, L. Valon, D. Navajas, J. M. García-Aznar, J. J. Muñoz, P. Roca-Cusachs and X. Trepat, Science, 2016, 353, 1157–1161 CrossRef CAS PubMed.
- D. Caballero, J. Comelles, M. Piel, R. Voituriez and D. Riveline, Trends Cell Biol., 2015, 25, 815–827 CrossRef PubMed.
- X. Sun, M. K. Driscoll, C. Guven, S. Das, C. A. Parent, J. T. Fourkas and W. Losert, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12557–12562 CrossRef CAS PubMed.
- M. Kim, J. W. Lim, H. J. Kim, S. K. Lee, S. J. Lee and T. Kim, Biosens. Bioelectron., 2015, 65, 257–264 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
