Open Access Article
Open Access ArticleAre two better than one? Comparing intermolecular and intramolecular indicator displacement assays in pyrophosphate sensors†
Xuejian
Liu
,
David G.
Smith
and
Katrina A.
Jolliffe
*
School of Chemistry, The University of Sydney, NSW 2006, Australia. E-mail: kate.jolliffe@sydney.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 2297
First published on 9th June 2016
Abstract
Peptide receptors with Zn(II)–DPA units and a covalently bound fluorescent coumarin indicator on an oxazole-containing scaffold are shown to function as more selective pyrophosphate sensors than the analogous chemosensing ensembles in indicator displacement assays.
Anions play crucial roles in biological processes. Pyrophosphate (P2O74−, PPi) for example, plays an important role in bioenergetics and metabolic processes. As such, the selective recognition and sensing of biologically relevant anions in water has numerous potential applications. For example, a sensor able to selectively detect pyrophosphate (PPi) in the presence of other phosphate species, especially nucleoside triphosphates, in aqueous solution would find use in bioanalytical applications.1–11
A number of recent studies have shown that receptors containing Zn(II)–dipicolylamino (DPA) units exhibit high selectivity and affinity towards phosphate oxoanions in water, and in some cases are able to discriminate between these species.1,12–15 We have previously reported oxazole-based cyclic peptide receptors with Zn(II)–DPA binding sites that show high levels of discrimination for PPi,16 as monitored through either colourimetric17 or fluorescent18,19 indicator displacement assays (IDAs). IDAs have been used successfully as a means of sensing anions in numerous chemosensing ensembles.9,20–25 However, for some applications it would be preferable to use sensors in which the indicator is covalently attached to the anion recognition moiety.
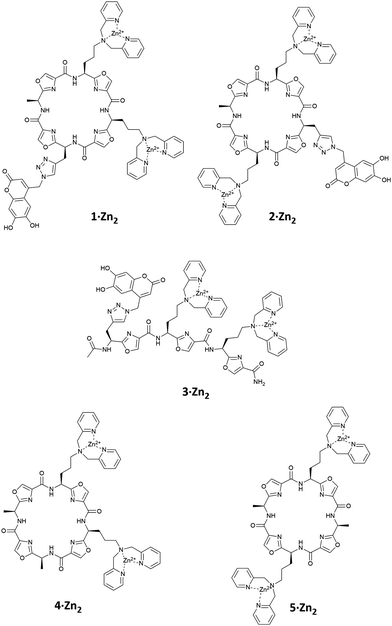
Intramolecular IDAs, in which the indicator is covalently attached to the receptor, provide a novel means by which to create molecular sensors.26 We report here a small family of oxazole-based peptides (1–3·Zn2), with Zn(II)–DPA anion binding sites, to which a fluorescent coumarin indicator is covalently bound through a flexible linker, so that it can act as an intramolecular fluorescent IDA (Fig. 1). We also compare the ability of these molecules to bind selectively to pyrophosphate with that of the analogous receptors in intermolecular IDAs with a comparable coumarin indicator.
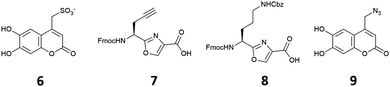
Based on our previous studies, in which we found that chemosensing ensembles comprising receptors 4·Zn2 or 5·Zn2 with a variety of indicators including coumarin sulfonate 6 (Fig. 2), showed good selectivity for pyrophosphate over other phosphate oxoanions in aqueous solution,9,17–19 we designed three receptors, 1·Zn2, 2·Zn2 and 3·Zn2 comprising an oxazole-containing peptide backbone with two side-chain appended Zn(II)–DPA binding sites and a coumarin fluorophore attached via a side-chain triazole moiety. 1·Zn2 and 2·Zn2 differ structurally in the order of the side chain appendages. In 1·Zn2 the Zn(II)–DPA units are adjacent to one another whereas in 2·Zn2 the coumarin is located in between them. 3·Zn2 is a linear analogue, designed to ascertain whether the cyclic peptide structure is required to maintain high selectivity.
Compounds 1–3 were prepared using methods similar to those previously described (see ESI† for full synthetic schemes).8,9,15,17,25,27 Briefly, the synthesis of the linear precursors was achieved using standard solid phase peptide synthesis techniques with the appropriate oxazole building blocks (7 and 8) (Fig. 2) and using Fmoc/HATU chemistry. The linear precursors to compounds 1 and 2 were prepared staring from 2-chlorotritylchloride resin, while for 3, the linear peptide was extended from Rink amide resin to provide the C-terminal amide upon cleavage from the resin. For the synthesis of 1 and 2, cleavage of the linear precursors from the resin was followed by solution phase cyclisation using the coupling reagent DMTMM·BF4. For all three peptides, the coumarin indicator was then attached via a copper(I)-catalysed azide–alkyne [3+2] cycloaddition (CuAAC) reaction28,29 with azide 9. The Cbz protecting groups were then removed using a solution of hydrogen bromide in acetic acid to give the corresponding diamines after basic workup. These were then subjected to reductive amination with 2-pyridinecarboxaldehyde using our previously established conditions, providing the DPA-functionalised cyclic peptide over two steps. Subsequent addition of two equivalents of Zn(NO3)2 gave the fully functionalised anion receptors, 1–3·Zn2.
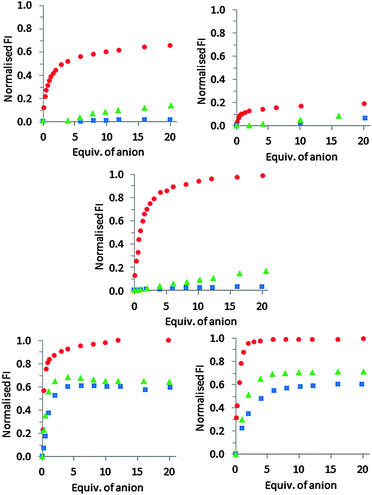
The anion binding behaviour of peptides 1–3·Zn2 was then assessed by titration and compared to that of chemosensing ensembles comprising either 4·Zn2 or 5·Zn2 and coumarin indicator 624 under the same conditions. PPi, ADP and ATP were incrementally added to a solution of 1–3·Zn2 in HEPES buffer (5 mM, 145 mM NaCl, pH 7.4) at 25 °C and the fluorescence emission recorded after each addition. In each case, addition of the anion resulted in an increase in fluorescence intensity, suggesting that binding of the anion resulted in displacement of the coumarin from the Zn(II)–DPA units with concomitant increase of fluorescence intensity (Fig. 3 and ESI†).
For receptor 1·Zn2, addition of PPi resulted in around a 6.5-fold fluorescent enhancement, accompanied by a 10 nm bathochromic shift (Fig. 4). Fitting of the titration data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model gave a log
1 binding model gave a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.2 as determined by a nonlinear least squares curve fitting. In contrast, addition of ADP or ATP induced only a minor fluorescence increase when added to 1·Zn2 (Fig. 3 and ESI†), indicating that 1·Zn2 exhibits a selective turn-on fluorescence response in the presence of PPi.
K = 5.2 as determined by a nonlinear least squares curve fitting. In contrast, addition of ADP or ATP induced only a minor fluorescence increase when added to 1·Zn2 (Fig. 3 and ESI†), indicating that 1·Zn2 exhibits a selective turn-on fluorescence response in the presence of PPi.
The response of receptor 2·Zn2 to PPi shows a similar 10 nm bathochromic shift, although the increase in emission intensity is much smaller compared to that observed for 1·Zn2, indicating 2·Zn2 is less sensitive towards PPi than 1·Zn2 (Fig. 3). In addition, similar fluorescent enhancements are observed for addition of ATP and ADP to 2·Zn2 as are observed for PPi addition, indicating that 2·Zn2 is less selective towards PPi over ADP and ATP than 1·Zn2.
These results suggest that the positioning of the two Zn(II)–DPA binding sites relative to the coumarin indicator on the cyclic peptide scaffold has a significant influence on anion binding capability. Positioning the indicator between the two Zn(II)–DPA units appears to make intramolecular indicator displacement more difficult, presumably as a result of stronger interactions between the coumarin and the Zn(II)–DPA units. This is attributed to the ability of the indicator to bridge both Zn(II)–DPA binding sites in this configuration. Positioning the two Zn(II)–DPA binding sites adjacent to each other on the peptide scaffold is required to achieve selective PPi binding in the intramolecular IDA, and may be attributed to indicator–metal interactions with only one of the Zn(II)–DPA units making the indicator easier to displace in 1·Zn2 than in 2·Zn2.
This is supported by the behaviour observed for 3·Zn2, in which the Zn(II)–DPA units are also adjacent to one another, and the coumarin occupies a terminal position. This shows a very similar overall profile with respect to the three anions as observed for 1·Zn2 (Fig. 3 and ESI†). There is a large fluorescent enhancement in the presence of PPi, but minimal changes in emission upon the addition of either ADP or ATP. The emission maxima for 3·Zn2 exhibits a blue shift upon PPi addition, compared to a red shift for 1·Zn2 and 2·Zn2. This results from the difference in the initial emission maxima, observed at 525 nm and 480 nm for 1·Zn2 and 3·Zn2, respectively which can be attributed to differences in the modes of binding from the coumarin indicator to the zinc(II) centres as a result of the different geometries of these molecules. Upon addition of PPi, both result in fluorescence with an emission maximum of 490 nm.
As previously observed for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5·Zn2 and coumarin indicator 6,18 addition of PPi, ATP and ADP to chemosensing ensembles of either 4·Zn2 or 5·Zn2 and 6 all resulted in indicator displacement with an observed increase in fluorescence (Fig. 3 and ESI†). Little difference was observed between 4·Zn2 and 5·Zn2 with similar binding affinities for all three anions observed for both chemosensing ensembles (apparent log
1 mixture of 5·Zn2 and coumarin indicator 6,18 addition of PPi, ATP and ADP to chemosensing ensembles of either 4·Zn2 or 5·Zn2 and 6 all resulted in indicator displacement with an observed increase in fluorescence (Fig. 3 and ESI†). Little difference was observed between 4·Zn2 and 5·Zn2 with similar binding affinities for all three anions observed for both chemosensing ensembles (apparent log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka for 5·Zn2 for PPi = 6.8; ATP = 6.3 and ADP = 6.0),18 and both 4·Zn2 and 5·Zn2 showed a slight preference for binding to PPi over ATP and ADP.
Ka for 5·Zn2 for PPi = 6.8; ATP = 6.3 and ADP = 6.0),18 and both 4·Zn2 and 5·Zn2 showed a slight preference for binding to PPi over ATP and ADP.
A comparison of the results for 1·Zn2 and 2·Zn2, with covalently attached indicators, to those obtained for 4·Zn2 and 5·Zn2, which use intermolecular indicator displacement assays to observe binding, suggests that by covalently attaching the coumarin derived indicator to the receptor a significant improvement in selectivity for PPi can be obtained, albeit with lower sensitivity. 1·Zn2 shows significantly higher selectivity for PPi compared to the analogous 4·Zn2, although the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka for PPi is an order of magnitude lower for 1·Zn2 compared to that observed for 4·Zn2. However, the improved selectivity requires the indicator to be attached at the correct position on the scaffold, with 2·Zn2 not displaying an improvement in either affinity or selectivity over the analagous indicator displacement approach with 5·Zn2. In terms of sensitivity, the titration data for both 1·Zn2 and 3·Zn2 indicate that a 5 μM solution of these receptors can detect PPi anions at low micromolar levels, whereas it has previously been suggested that 5·Zn2 is capable of detection of nanomolar concentrations of PPi.19
Ka for PPi is an order of magnitude lower for 1·Zn2 compared to that observed for 4·Zn2. However, the improved selectivity requires the indicator to be attached at the correct position on the scaffold, with 2·Zn2 not displaying an improvement in either affinity or selectivity over the analagous indicator displacement approach with 5·Zn2. In terms of sensitivity, the titration data for both 1·Zn2 and 3·Zn2 indicate that a 5 μM solution of these receptors can detect PPi anions at low micromolar levels, whereas it has previously been suggested that 5·Zn2 is capable of detection of nanomolar concentrations of PPi.19
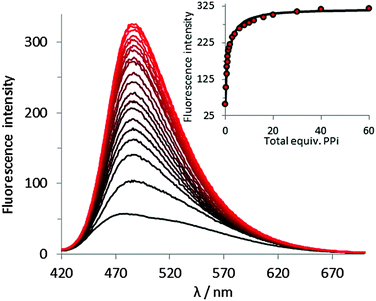
One potential application of fluorescent PPi sensors is to monitor enzymatic processes. Pyrophosphatase is an enzyme which catalyses the hydrolysis of PPi to two phosphate ions in the presence of magnesium. To verify the general capability of our peptide-derived PPi sensors for real time monitoring of enzymatic processes, a fluorescence assay was used to monitor pyrophosphatase activity.
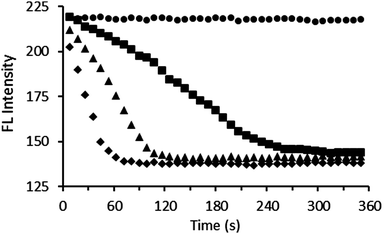
Pyrophosphatase was added to a solution containing either 1·Zn2 or 3·Zn2, and excess PPi in Tris buffer at 30 °C. Differing quantities of PPi were used with each sensor, based upon the differing sensitivities exhibited by the two sensors. The fluorescence response was then measured at regular time intervals. Addition of pyrophosphatase resulted in a decrease in fluorescence intensity (emission was monitored at 485 nm), with a more rapid decrease observed in the presence of larger quantities of enzyme (Fig. 5). This is consistent with a reduction in PPi concentration as the enzyme binds to and breaks down the PPi. The coumarin can then bind to the zinc centres of 1·Zn2 or 3·Zn2, resulting in quenching of the fluorescence emission. These enzyme assay results suggest the general applicability of peptide PPi sensors for monitoring the enzymatic process of inorganic pyrophosphatase.
In summary, we have developed oxazole-based peptide receptors which incorporate a covalently linked fluorescent coumarin reporter. This allows the receptor to function as an intramolecular indicator displacement assay, and to selectivity detect PPi. Compared to an intermolecular analogue, we observed greatly enhanced selectivity for PPi over competing phosphate species using systems with covalently attached indicators. We have demonstrated an application of these receptors to measure pyrophosphatase enzyme activity in real time.
We thank the ARC (DP110100682 and DP140100227) for financial support.
Notes and references
- S. Lee, K. K. Y. Yuen, K. A. Jolliffe and J. Yoon, Chem. Soc. Rev., 2015, 44, 1749–1762 RSC.
- J. F. Zhang, S. Kim, J. H. Han, S. J. Lee, T. Pradhan, Q. Y. Cao, S. J. Lee, C. Kang and J. S. Kim, Org. Lett., 2011, 13, 5294–5297 CrossRef CAS PubMed.
- W. H. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362–1365 CrossRef CAS PubMed.
- N. L. Han, K. M. K. Swamy, K. K. Sook, J. Y. Kwon, Y. Kim, S. J. Kim, J. Y. Yeo and J. Yoon, Org. Lett., 2007, 9, 243–246 CrossRef PubMed.
- D. H. Lee, S. Y. Kim and J. I. Hong, Angew. Chem., Int. Ed., 2004, 43, 4777–4780 CrossRef CAS PubMed.
- J. H. Lee, A. R. Jeong, J. H. Jung, C. M. Park and J. I. Hong, J. Org. Chem., 2011, 76, 417–423 CrossRef CAS PubMed.
- H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and J. Yoon, J. Am. Chem. Soc., 2007, 129, 3828–3829 CrossRef CAS PubMed.
- K. K. Y. Yuen and K. A. Jolliffe, Chem. Commun., 2013, 49, 4824–4826 RSC.
- S. J. Butler and K. A. Jolliffe, Org. Biomol. Chem., 2011, 9, 3471–3483 CAS.
- S. K. Kim, D. H. Lee, J.-I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23–31 CrossRef CAS PubMed.
- W. Zhu, X. Huang, Z. Guo, X. Wu, H. Yu and H. Tian, Chem. Commun., 2012, 48, 1784–1786 RSC.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC.
- A. Ojida, Y. Mito-oka, K. Sada and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 2454–2463 CrossRef CAS PubMed.
- T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141–152 RSC.
- S. J. Butler, K. A. Jolliffe, W. Y. G. Lee, M. J. McDonough and A. J. Reynolds, Tetrahedron, 2011, 67, 1019–1029 CrossRef CAS.
- R. B. P. Elmes and K. A. Jolliffe, Chem. Commun., 2015, 51, 4951–4968 RSC.
- X. Liu, H. T. Ngo, Z. Ge, S. J. Butler and K. A. Jolliffe, Chem. Sci., 2013, 4, 1680–1686 RSC.
- S. J. Butler and K. A. Jolliffe, Chem. – Asian J., 2012, 7, 2621–2628 CrossRef CAS PubMed.
- M. J. McDonough, A. J. Reynolds, W. Y. G. Lee and K. A. Jolliffe, Chem. Commun., 2006, 2971–2973 RSC.
- S. L. Wiskur, P. N. Floriano, E. V. Anslyn and J. T. McDevitt, Angew. Chem., Int. Ed., 2003, 42, 2070–2072 CrossRef CAS PubMed.
- B. P. Morgan, S. He and R. C. Smith, Inorg. Chem., 2007, 46, 9262–9266 CrossRef CAS PubMed.
- M. K. Coggins, A. M. Parker, A. Mangalum, G. A. Galdamez and R. C. Smith, Eur. J. Org. Chem., 2009, 343–348 CrossRef CAS.
- X. Sun, K. Lacina, E. C. Ramsamy, S. E. Flower, J. S. Fossey, X. Qian, E. V Anslyn, S. D. Bull and T. D. James, Chem. Sci., 2015, 6, 2963–2967 RSC.
- R. G. Hanshaw, S. M. Hilkert, H. Jiang and B. D. Smith, Tetrahedron Lett., 2004, 45, 8721–8724 CrossRef CAS.
- V. E. Zwicker, X. Liu, K. K. Y. Yuen and K. A. Jolliffe, Supramol. Chem., 2016, 28, 192–200 CrossRef CAS.
- T. Minami, Y. Liu, A. Akdeniz, P. Koutnik, N. A. Esipenko, R. Nishiyabu, Y. Kubo and P. Anzenbacher, J. Am. Chem. Soc., 2014, 136, 11396–11401 CrossRef CAS PubMed.
- V. E. Zwicker, B. M. Long and K. A. Jolliffe, Org. Biomol. Chem., 2015, 13, 7822–7829 CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and NMR spectra for new compounds, experimental procedures, anion titration data, pyrophosphatase assay data. See DOI: 10.1039/c6cc03680e |
| This journal is © The Royal Society of Chemistry 2016 |