Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed borylative coupling of vinylazaarenes and N-Boc imines†
Joshua J.
Smith
ab,
Daniel
Best
ab and
Hon Wai
Lam
*ab
aEaStCHEM, School of Chemistry, University of Edinburgh, Joseph Black Building, The King's Buildings, David Brewster Road, Edinburgh, EH9 3FJ, UK
bSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. E-mail: hon.lam@nottingham.ac.uk
First published on 17th February 2016
Abstract
Cu-catalyzed three-component couplings of vinylazaarenes, B2(pin)2, and N-Boc imines are described. Oxidation of the initially formed boronate gives azaarene-containing, Boc-protected amino alcohols with reasonable to good diastereoselectivities.
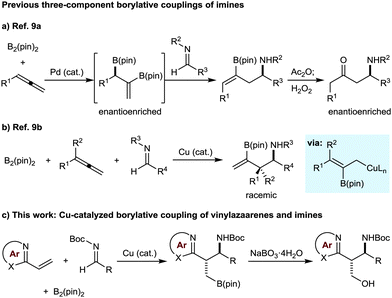
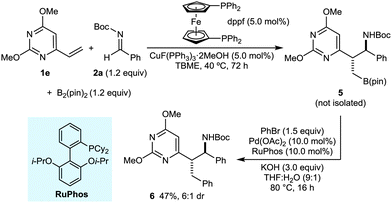
Nitrogen-containing aromatic heterocycles (azaarenes) and α-branched amines are common structures in biologically active molecules. New catalytic reactions that can assemble compounds containing both of these motifs in a convergent and stereocontrolled manner are therefore highly valuable.1 In this context, we have described enantioselective Pd-catalyzed additions of alkylazaarenes to N-Boc imines,2 and enantioselective Cu-catalyzed reductive couplings of vinylazaarenes with N-Boc imines.3 However, the integration of such coupling reactions with the simultaneous introduction of another, versatile functional group that can be exploited in subsequent manipulations would open up additional synthetic possibilities. Given the widely recognized utility of organoboron compounds,4 and in connection with our interest in the use of azaarenes as activating groups in catalytic reactions,5–7 we were prompted to examine the borylative coupling of vinylazaarenes with imines. Precedent for such a transformation is limited; although numerous borylative three-component coupling reactions have been described,8,9 none employ alkenylazaarenes, and to our knowledge, only two examples employ imines (Scheme 1a and b).9 The Morken group has reported enantioselective Pd-catalyzed diborations of allenes, followed by the addition of imines to the resulting bisboronates to give homoallylic amines (Scheme 1a).9a After acetylation of the amine and oxidation, β-acetamidoketones were isolated in good yields and high enantioselectivities.9a Recently, Procter and co-workers described Cu-catalyzed borylative couplings of allenes with imines that give branched homoallylic amines containing an alkenylboronate (Scheme 1b).9b Extension of these processes to other substrate classes would lead to important increases in reaction scope. Herein, we report the first examples of Cu-catalyzed borylative couplings of vinylazaarenes with N-Boc imines to give highly functionalized heterocycle-containing building blocks (Scheme 1b).10,11
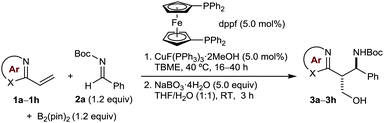
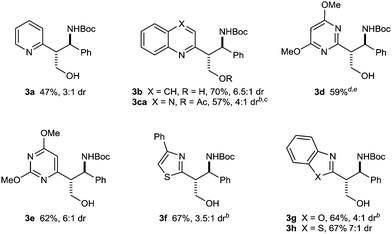
Our investigation began with a search for effective conditions for the three-component coupling of various vinylazaarenes and aldimines with bis(pinacolato)diboron. From these studies, we found that stirring a solution of the vinylazaarene (1.0 equiv.), N-Boc aldimine (1.2 equiv.), and B2(pin)2 (1.2 equiv.) in TBME at 40 °C in the presence of CuF(PPh)3·2MeOH (5.0 mol%) and dppf (5.0 mol%) successfully gave borylative coupling products with high conversions.12–14 These products undergo decomposition on silica gel, and were therefore oxidized to the corresponding chromatographically stable primary alcohols with NaBO3·4H2O.15 Under these conditions, a range of vinylazaarenes 1a–1h underwent smooth reaction with the N-Boc imine 2a derived from benzaldehyde to give azaarene-containing, Boc-protected amino alcohols 3a, 3b, and 3d–3h (Table 1). Products containing pyridine (3a), quinoline (3b), one of two different dimethoxypyrimidines (3d and 3e), 5-phenylthiazole (3f), benzoxazole (3g), or benzothiazole (3h) groups were prepared in reasonable to good yields and diastereoselectivities (up to 73% yield over two steps and up to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). The relative configurations of the products were assigned by analogy with those of 3b and 3h, which were determined by X-ray crystallography to be the anti-diastereomers.16 When 2-vinylquinoxaline was employed as the substrate, acetylation of the primary alcohol 3c was performed to give 3ca to facilitate separation from pinacol, a byproduct of the boronate oxidation.15
1 dr). The relative configurations of the products were assigned by analogy with those of 3b and 3h, which were determined by X-ray crystallography to be the anti-diastereomers.16 When 2-vinylquinoxaline was employed as the substrate, acetylation of the primary alcohol 3c was performed to give 3ca to facilitate separation from pinacol, a byproduct of the boronate oxidation.15
| a Reactions were conducted using 0.50 mmol of vinylazaarene in TBME (1.25 mL). Yields are of isolated single diastereomers unless otherwise stated. Diastereomeric ratios were determined by 1H NMR analysis of the unpurified reaction mixtures. b Isolated as a mixture of diastereomers in the same ratio as in the unpurified reaction mixture. c Unpurified 3c was treated with Ac2O/pyridine to give 3ca to facilitate purification. d Reaction performed at 60 °C. e Overlapping signals precluded accurate determination of dr by 1H NMR analysis. |
|---|
|
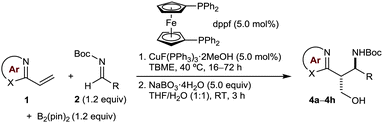
Table 2 presents the results of borylative coupling of various vinylazaarenes with a range of N-Boc aldimines, which gave products with diastereoselectivities ranging from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 8) to 8.5
1 dr (entry 8) to 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 4). Imines derived from benzaldehydes containing methyl (entries 2, 7, and 9), fluoro (entries 1 and 11), chloro (entry 3), bromo (entry 5), or methoxy substituents (entries 4 and 8) at various positions of the aromatic ring are compatible with this process. The N-Boc imine derived from cyclohexane carboxaldehyde also successfully underwent borylative coupling, although with moderate conversion and diastereoselectivity (entry 12).
1 dr (entry 4). Imines derived from benzaldehydes containing methyl (entries 2, 7, and 9), fluoro (entries 1 and 11), chloro (entry 3), bromo (entry 5), or methoxy substituents (entries 4 and 8) at various positions of the aromatic ring are compatible with this process. The N-Boc imine derived from cyclohexane carboxaldehyde also successfully underwent borylative coupling, although with moderate conversion and diastereoselectivity (entry 12).
| Entry | Product | Yieldb (%) | drc | |
|---|---|---|---|---|
a Reactions were conducted using 0.50 mmol of vinylazaarene in TBME (1.25 mL).
b Yield of isolated products.
c Diastereomeric ratios were determined by 1H NMR analysis of the unpurified reaction mixtures. Ratios in parentheses are those of the isolated product. Where ratios in parentheses are absent, the products were isolated in the same ratio as in unpurified reaction mixture.
d A second fraction consisting of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers was also obtained in 17% yield. 1 mixture of diastereomers was also obtained in 17% yield.
|
||||
| 1 |
|
4a | 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (>19 1 (>19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 2 |
|
4b Ar = 3-MeC6H4 | 83 | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 4c Ar = 3-ClC6H4 | 60 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 1 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 4 | 4d Ar = 4-MeOC6H4 | 58 | 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (18 1 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 5 | 4e Ar = 4-BrC6H4 | 60 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 1 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 6 | 4f Ar = 2-naphthyl | 56 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (11 1 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 7 | 4g Ar = 2-MeC6H6 | 41d | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (>19 1 (>19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
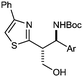
| 8 |
|
4h Ar = 3-MeOC6H4 | 58 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 1 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 | 4i Ar = 4-MeC6H4 | 69 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (4 1 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
| 10 | 4j Ar = 4-MeOC6H4 | 60 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| 11 | 4k Ar = 4-FC6H4 | 64 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
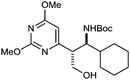
| 12 |
|
4l | 37 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (>19 1 (>19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
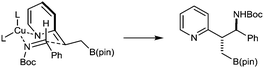
A tentative stereochemical model that could account for the anti-selectivity of these reactions involves the addition of an E-azaallylcopper species to the imine in a chair-like transition state (Fig. 1). However, we cannot exclude alternative models involving boat-like structures or open transition states.
To further demonstrate the synthetic utility of the borylative coupling products, the reaction of vinylpyrimidine 1e and N-Boc imine 2a was repeated to give alkylboronate 5. Without isolation, 5 was reacted with bromobenzene under Suzuki–Miyaura cross-coupling conditions described previously17 using RuPhos18 as the ligand, to give the phenylated product 6 in 47% yield over two steps as a single diastereoisomer (crude dr 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 2).19,20
1) (Scheme 2).19,20
The Boc group of the products may be removed under acidic conditions, as demonstrated by the deprotection of 3b using TMSCl in MeOH,2,3 which provided the bishydrochloride salt 7 in >99% yield (eqn (1)).
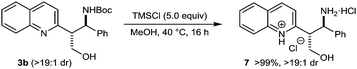 | (1) |
In conclusion, we have demonstrated the utility of vinylazaarenes as substrates for copper-catalyzed borylative couplings with N-Boc imines. The reactions provide, after oxidation of the initially formed alkylboronates, azaarene-containing, Boc-protected amino alcohols with moderate-to-good diastereoselectivities. Future work will be focused on the development of enantioselective variants of this process.21
We thank the EPSRC (Industrial CASE studentship to J. J. S. and Leadership Fellowship to H. W. L, grant no. EP/I004769/1 and EP/I004769/2), GlaxoSmithKline, and the ERC (Starting Grant No. 258580) for financial support. We thank Dr. William Lewis at the University of Nottingham for X-ray crystallography, and Dr. Alan Nadin (GlaxoSmithKline) for helpful discussions.
Notes and references
- For reviews of catalytic enantioselective Friedel–Crafts additions of electron-rich azaarenes to imines or enamides, see: (a) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903–2915 CrossRef CAS PubMed; (b) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed; (c) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190–2201 RSC; (d) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449–4465 RSC; (e) V. Terrasson, R. M. de Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635–2655 CrossRef CAS; (f) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS PubMed.
- D. Best, S. Kujawa and H. W. Lam, J. Am. Chem. Soc., 2012, 134, 18193–18196 CrossRef CAS PubMed.
- B. Choi, A. Saxena, J. J. Smith, G. H. Churchill and H. W. Lam, Synlett, 2015, 350–351 CAS.
- For reviews, see: (a) Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, ed. D. G. Hall, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2nd edn, 2011, vol. 1 and 2 Search PubMed; (b) D. S. Matteson, J. Org. Chem., 2013, 78, 10009–10023 CrossRef CAS PubMed; (c) D. Leonori and V. K. Aggarwal, Acc. Chem. Res., 2014, 47, 3174–3183 CrossRef CAS PubMed.
- D. Best and H. W. Lam, J. Org. Chem., 2014, 79, 831–845 CrossRef CAS PubMed.
- For previous work from our group on C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-containing azaarenes as activating groups in enantioselective catalysis, see:
(a) L. Rupnicki, A. Saxena and H. W. Lam, J. Am. Chem. Soc., 2009, 131, 10386–10387 CrossRef CAS PubMed;
(b) G. Pattison, G. Piraux and H. W. Lam, J. Am. Chem. Soc., 2010, 132, 14373–14375 CrossRef CAS PubMed;
(c) A. Saxena and H. W. Lam, Chem. Sci., 2011, 2, 2326–2331 RSC;
(d) A. Saxena, B. Choi and H. W. Lam, J. Am. Chem. Soc., 2012, 134, 8428–8431 CrossRef CAS PubMed;
(e) C. Fallan and H. W. Lam, Chem. – Eur. J., 2012, 18, 11214–11218 CrossRef CAS PubMed;
(f) I. D. Roy, A. R. Burns, G. Pattison, B. Michel, A. J. Parker and H. W. Lam, Chem. Commun., 2014, 50, 2865–2868 RSC.
N-containing azaarenes as activating groups in enantioselective catalysis, see:
(a) L. Rupnicki, A. Saxena and H. W. Lam, J. Am. Chem. Soc., 2009, 131, 10386–10387 CrossRef CAS PubMed;
(b) G. Pattison, G. Piraux and H. W. Lam, J. Am. Chem. Soc., 2010, 132, 14373–14375 CrossRef CAS PubMed;
(c) A. Saxena and H. W. Lam, Chem. Sci., 2011, 2, 2326–2331 RSC;
(d) A. Saxena, B. Choi and H. W. Lam, J. Am. Chem. Soc., 2012, 134, 8428–8431 CrossRef CAS PubMed;
(e) C. Fallan and H. W. Lam, Chem. – Eur. J., 2012, 18, 11214–11218 CrossRef CAS PubMed;
(f) I. D. Roy, A. R. Burns, G. Pattison, B. Michel, A. J. Parker and H. W. Lam, Chem. Commun., 2014, 50, 2865–2868 RSC. - For examples of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-containing azaarenes as activating groups in enantioselective catalysis from other groups, see:
(a) B. M. Trost and D. A. Thaisrivongs, J. Am. Chem. Soc., 2008, 130, 14092–14093 CrossRef CAS PubMed;
(b) B. M. Trost and D. A. Thaisrivongs, J. Am. Chem. Soc., 2009, 131, 12056–12057 CrossRef CAS PubMed;
(c) B. M. Trost, D. A. Thaisrivongs and J. Hartwig, J. Am. Chem. Soc., 2011, 133, 12439–12441 CrossRef CAS PubMed;
(d) S. Vera, Y. K. Liu, M. Marigo, E. C. Escudero-Adan and P. Melchiorre, Synlett, 2011, 489–494 CAS;
(e) T. Li, J. Zhu, D. Wu, X. Li, S. Wang, H. Li, J. Li and W. Wang, Chem. – Eur. J., 2013, 19, 9147–9150 CrossRef CAS PubMed;
(f) M. Meazza, V. Ceban, M. B. Pitak, S. J. Coles and R. Rios, Chem. – Eur. J., 2014, 20, 16853–16857 CrossRef CAS PubMed.
N-containing azaarenes as activating groups in enantioselective catalysis from other groups, see:
(a) B. M. Trost and D. A. Thaisrivongs, J. Am. Chem. Soc., 2008, 130, 14092–14093 CrossRef CAS PubMed;
(b) B. M. Trost and D. A. Thaisrivongs, J. Am. Chem. Soc., 2009, 131, 12056–12057 CrossRef CAS PubMed;
(c) B. M. Trost, D. A. Thaisrivongs and J. Hartwig, J. Am. Chem. Soc., 2011, 133, 12439–12441 CrossRef CAS PubMed;
(d) S. Vera, Y. K. Liu, M. Marigo, E. C. Escudero-Adan and P. Melchiorre, Synlett, 2011, 489–494 CAS;
(e) T. Li, J. Zhu, D. Wu, X. Li, S. Wang, H. Li, J. Li and W. Wang, Chem. – Eur. J., 2013, 19, 9147–9150 CrossRef CAS PubMed;
(f) M. Meazza, V. Ceban, M. B. Pitak, S. J. Coles and R. Rios, Chem. – Eur. J., 2014, 20, 16853–16857 CrossRef CAS PubMed. - For selected examples of borylative three-component coupling reactions not involving imines, see: (a) H. Y. Cho and J. P. Morken, J. Am. Chem. Soc., 2008, 130, 16140–16141 CrossRef CAS PubMed; (b) S. Mannathan, M. Jeganmohan and C.-H. Cheng, Angew. Chem., Int. Ed., 2009, 48, 2192–2195 CrossRef CAS PubMed; (c) H. Y. Cho and J. P. Morken, J. Am. Chem. Soc., 2010, 132, 7576–7577 CrossRef CAS PubMed; (d) H. Y. Cho, Z. Yu and J. P. Morken, Org. Lett., 2011, 13, 5267–5269 CrossRef CAS PubMed; (e) A. Welle, V. Cirriez and O. Riant, Tetrahedron, 2012, 68, 3435–3443 CrossRef CAS; (f) F. Meng, H. Jang, B. Jung and A. H. Hoveyda, Angew. Chem., Int. Ed., 2013, 52, 5046–5051 CrossRef CAS PubMed; (g) F. Meng, K. P. McGrath and A. H. Hoveyda, Nature, 2014, 513, 367–374 CrossRef CAS PubMed; (h) K. Semba, N. Bessho, T. Fujihara, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2014, 53, 9007–9011 CrossRef CAS PubMed; (i) F. Meng, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 11304–11307 CrossRef CAS PubMed.
- For borylative three-component coupling reactions of allenes and imines, see: (a) J. D. Sieber and J. P. Morken, J. Am. Chem. Soc., 2006, 128, 74–75 CrossRef CAS PubMed; (b) J. Rae, K. Yeung, J. J. W. McDouall and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 1102–1107 CrossRef CAS PubMed.
- For Cu-catalyzed three-component borylative aldol reactions of α,β-unsaturated carbonyl compounds with carbonyl compounds, see ref. 8e.
- For examples of biologically active compounds containing a 2-(aminoalkyl)azaarene substructure, which are produced using the methodology described herein, see references cited within ref. 2.
- In several cases, increasing the temperature significantly above 40 °C led to appreciable quantities of the products resulting from 1,4-boration of the vinylazaarene without coupling to the imine. Attempts to decrease the reaction times by increasing the concentration led to solubility problems.
- Other bidentate ligands examined included DPEphos, dppe, dppbz, and bipy. No significant impact on the rate of the reaction was observed, but compared with dppf, these ligands gave products in poorer diastereomeric ratios. Using CuF(PPh)3·2MeOH without an additional bidentate ligand gave low yields of borylative coupling products in poor diastereomeric ratios, along with significant quantities of products resulting from 1,4-boration of the vinylazaarene without coupling to the imine.
- Control reactions conducted in the absence of the copper salt, but using PPh3 (15.0 mol%) or dppf (5.0 mol%), with or without MeOH (10.0 mol% or 5.0 equiv.), did not provide any products. For PPh3- or dppf-catalyzed 1,4-boration of α,β-unsaturated ketones, see: A. Bonet, H. Gulyás and E. Fernández, Angew. Chem., Int. Ed., 2010, 49, 5130–5134 CrossRef CAS PubMed.
- C. N. Farthing and S. P. Marsden, Tetrahedron Lett., 2000, 41, 4235–4238 CrossRef CAS.
- See the ESI†.
- S. N. Mlynarski, C. H. Schuster and J. P. Morken, Nature, 2014, 505, 386–390 CrossRef CAS PubMed.
- M. D. Charles, P. Schultz and S. L. Buchwald, Org. Lett., 2005, 7, 3965–3968 CrossRef CAS PubMed.
- For other examples of Suzuki–Miyaura coupling reactions of non-benzylic, non-allylic, alkyl pinacol boronates, see ref. 8e, 17 and: (a) M. Sato, N. Miyaura and A. Suzuki, Chem. Lett., 1989, 1405–1408 CrossRef CAS; (b) G. Zou and J. R. Falck, Tetrahedron Lett., 2001, 42, 5817–5819 CrossRef CAS; (c) J. D. Lawrence, M. Takahashi, C. Bae and J. F. Hartwig, J. Am. Chem. Soc., 2004, 126, 15334–15335 CrossRef CAS PubMed; (d) C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528–532 CrossRef CAS PubMed; (e) R. Sakae, N. Matsuda, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 1228–1231 CrossRef CAS PubMed; (f) L. Zhang, Z. Zuo, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2014, 53, 2696–2700 CrossRef CAS PubMed.
- For Suzuki–Miyaura coupling of primary alkyltrifluoroborates, see: S. D. Dreher, S.-E. Lim, D. L. Sandrock and G. A. Molander, J. Org. Chem., 2009, 74, 3626–3631 CrossRef CAS PubMed.
- Thus far, our preliminary efforts at developing an enantioselective variant of these reactions have given only low enantiomeric excesses.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data for new compounds, and crystallographic data for 3b and 3h. CCDC 1431894 and 1431895. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc00603e |
| This journal is © The Royal Society of Chemistry 2016 |