Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new type of oxidative addition of an iodoarene to a Pd(II) complex†
Inmaculada
Vicente-Hernández
a,
María-Teresa
Chicote
a,
José
Vicente
*a and
Delia
Bautista
b
aGrupo de Química Organometálica, Departamento de Química Inorgánica, Facultad de Química, Universidad de Murcia, Apartado 4021, Murcia, 30071, Spain. E-mail: jvs1@um.es; mch@um.es
bSAI, Universidad de Murcia, Spain. E-mail: dbc@um.es
First published on 2nd November 2015
Abstract
Oxidative addition of N-(2-iodophenyl)formamide to Pd(dba)2 (dba = dibenzylideneacetone) in the presence of 4,4′-ditertbutyl-2,2′-bipyridine (tBubpy) produces [Pd(C6H4NHCHO-2)I(tBubpy)] (1) which inserts 2-iodophenyl isocyanide to give [Pd{C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4I-2)C6H4NHCHO-2}I(tBubpy)] (2). Dehydroiodination of 2 with Tl(acac) (acacH = acetylacetone) gives the stable Pd(IV) complex OC-6-35-[Pd{C,N,N–C(
NC6H4I-2)C6H4NHCHO-2}I(tBubpy)] (2). Dehydroiodination of 2 with Tl(acac) (acacH = acetylacetone) gives the stable Pd(IV) complex OC-6-35-[Pd{C,N,N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4-2)C6H4NCHO-2}I(tBubpy)] (4) likely resulting from the spontaneous oxidative addition of the I–Ar moiety present in the unstable intermediate Pd(II) complex [Pd{C,N–C(
NC6H4-2)C6H4NCHO-2}I(tBubpy)] (4) likely resulting from the spontaneous oxidative addition of the I–Ar moiety present in the unstable intermediate Pd(II) complex [Pd{C,N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4I-2)C6H4NCHO-2}(tBubpy)] (3). The crystal structure of 4 shows various C–H⋯O hydrogen bonds resulting in chains of dimers stacked along the a axis.
NC6H4I-2)C6H4NCHO-2}(tBubpy)] (3). The crystal structure of 4 shows various C–H⋯O hydrogen bonds resulting in chains of dimers stacked along the a axis.
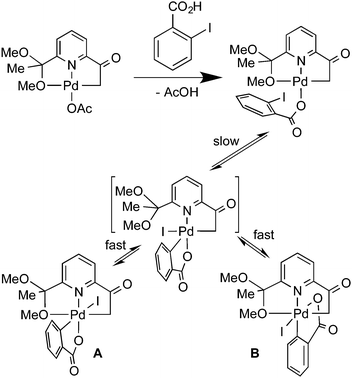
The important role of Pd-catalyzed C–C cross coupling reactions in organic synthesis is very well known.1 These reactions involve Pd(0)2,3 or Pd(II)4 precatalysts and, in most cases, the catalyst is Pd metal or a Pd(0) complex formed from the precatalyst. However, in some cases in which the precatalyst is a Pd(II) complex, experimental3,5,6 and theoretical7 studies suggested a Pd(II)/Pd(IV) catalytic cycle as an alternative to the usual Pd(0)/Pd(II) one. The main objections to this proposal, when an aryl halide is involved (Heck, Suzuki–Miyaura, Sonogashira, Stille, etc. coupling reactions), were that (1) the necessary oxidative addition of the haloarene to Pd(II) had no experimental support, and (2) the Pd(IV) intermediate formed during catalysis had not been detected. However, we have recently given experimental evidence against these two objections by isolating the Pd(IV) oxidative addition product (A, Scheme 1) of an aryl iodide to a Pd(II) complex,8,9 and detecting the Pd(IV) complex in a Heck-type catalytic reaction.10
The easy formation of complexes A and B (Scheme 1) was explained as a consequence of the coordination of the 2-iodobenzoato ligand that would bring the iodine and Pd atoms close to each other.9 We have used the same coordination-assisted oxidative addition of the 2-iodobenzoato ligand with other Pd(II) complexes.8
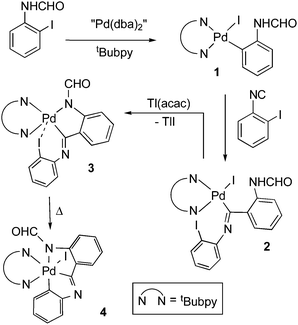
In this work we describe a new way of assistance of the oxidative addition of an aryl halide to a Pd(II) complex. It involves the insertion of an isocyanide into the Pd–C bond of an aryl Pd(II) complex (1 in Scheme 2), a very well known process11 that has been recently reviewed.12 The rising interest of Pd(IV) chemistry in synthesis and catalysis is very well established.13
Complex 1 (Scheme 2) was obtained by the oxidative addition of N-(2-iodophenyl)formamide14 to Pd2(dba)3·dba (“Pd(dba)2”, dba = dibenzylideneacetone) in the presence of tBubpy (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, toluene, room temperature, 5 h, 80%). Insertion of 2-iodophenyl isocyanide into the Pd–C bond of 1 produced the iminobenzoyl complex 2 (1
1, toluene, room temperature, 5 h, 80%). Insertion of 2-iodophenyl isocyanide into the Pd–C bond of 1 produced the iminobenzoyl complex 2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, CH2Cl2, room temperature, 20 min, 89%) which decomposed upon heating in solution or even upon standing at room temperature to give the almost quantitative amount of [PdI2(tBubpy)] along with an unresolved mixture of products. This result suggested that an unstable diiodopalladium(IV) complex, probably [Pd{C,N,N–C(
1, CH2Cl2, room temperature, 20 min, 89%) which decomposed upon heating in solution or even upon standing at room temperature to give the almost quantitative amount of [PdI2(tBubpy)] along with an unresolved mixture of products. This result suggested that an unstable diiodopalladium(IV) complex, probably [Pd{C,N,N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4-2)C6H4NHCHO-2}I2(tBubpy)], formed from the oxidative addition to Pd(II) of the I-iminobenzoyl fragment. The great instability of the only known diiodo Pd(IV) organometallic complex has been previously reported.15 In order to avoid this decomposition process we decided to dehydroiodinate complex 2 with Tl(acac) (acacH = acetylacetone) but, instead of the expected complex 3, spontaneous formation of the Pd(IV) complex 4 occurred, containing a stabilizing6,16 pincer ligand. The isolated reaction mixture (1
NC6H4-2)C6H4NHCHO-2}I2(tBubpy)], formed from the oxidative addition to Pd(II) of the I-iminobenzoyl fragment. The great instability of the only known diiodo Pd(IV) organometallic complex has been previously reported.15 In order to avoid this decomposition process we decided to dehydroiodinate complex 2 with Tl(acac) (acacH = acetylacetone) but, instead of the expected complex 3, spontaneous formation of the Pd(IV) complex 4 occurred, containing a stabilizing6,16 pincer ligand. The isolated reaction mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, acetone, 1 h, room temperature) was shown by 1H NMR to contain mainly 4, while intermediate 3, which we assume to be a minor component of the mixture, could not be isolated even from a 15 min reaction. Pure complex 4 was isolated in 75% yield at 50 °C for 5 h but also after a couple of days standing at room temperature.
1, acetone, 1 h, room temperature) was shown by 1H NMR to contain mainly 4, while intermediate 3, which we assume to be a minor component of the mixture, could not be isolated even from a 15 min reaction. Pure complex 4 was isolated in 75% yield at 50 °C for 5 h but also after a couple of days standing at room temperature.
The oxidative addition reactions of R–X (R = alkyl) compounds to Pd(II) complexes lead to cis-, trans- or mixtures of both Pd(IV) isomers depending on the nature of R.17 In the case of the oxidative addition of 2-iodobenzoate (Scheme 1), the 1H NMR spectrum of the reaction mixture showed an equilibrium between both isomers A and B, the later characterized by X-ray crystallography. The presence of a weakly coordinating group (MeO) may be responsible for the easy conversion of both isomers through a pentacoordinate intermediate,9 which in the present case seems not to be available (even at 50 °C for 5 h) since both the tBubpy and the C∧N ligands form robust palladacycles. Therefore, the results of the three known reported cases suggest that the intramolecular oxidative addition of aryl iodides to Pd(II) leads to the cis isomer that can isomerize to the trans isomer if a weakly coordinating group facilitates the process.
According to NMR data, complexes 1 and 2 form as mixtures of isomers as is also the case of the starting compound N-(2-iodophenyl)formamide.14,18
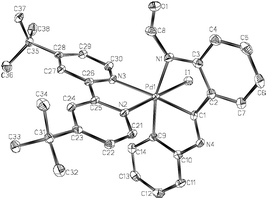
The crystal structure of 4·CH2Cl2 (Fig. 1) has been determined by X-ray diffraction methods. It shows the Pd atom in a rather distorted octahedral environment. The greater trans influence of carbon with respect to nitrogen donor ligands is observed in the Pd–NtBubpy bond distances (2.1763(18) vs. 2.0767(19) Å). The Pd–I bond distance (2.5992(3) Å) is similar to that in the only other monoiodo Pd(IV) complex structurally characterized having iodo trans to nitrogen (2.5902(3) Å).19 A chain of dimers forms along the a axis through various C–H⋯O hydrogen bonds.
Very few crystal structures of Pd(IV)-iodo complexes have been reported, namely those of one triiodo20 and three monoiodo9,19,21 derivatives.
In conclusion, we show an alternative way to oxidatively add an iodoarene to a Pd(II) complex to give a very stable aryl Pd(IV) complex through insertion of 2-iodophenyl isocyanide into the C–Pd bond of an aryl Pd(II) complex. This work opens a new research line that, provided the appropriate aryl Pd(II) complexes and 2-iodoarylisocyanides (or related reagents) were used, could lead to interesting organic products through unstable Pd(IV) intermediates.
We thank the Ministerio de Ciencia e Innovación (Spain), FEDER (Project CTQ2011-24016), and Fundación Séneca (CARM, Murcia, Spain; 04539/GERM/06) for financial support.
Notes and references
- X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed; A. Zapf and M. Beller, Chem. Commun., 2005, 431–440 RSC; W. A. Herrmann, C. Brossmer, K. Öfele, C. P. Reisinger, T. Priermeier, M. Beller and H. Fischer, Angew. Chem., Int. Ed. Engl., 1995, 34, 1844–1848 CrossRef.
- C. Amatore and A. Jutand, Acc. Chem. Res., 2000, 33, 314–321 CrossRef CAS PubMed; G. T. Crisp, Chem. Soc. Rev., 1998, 27, 427–436 RSC.
- L. Xue and Z. Lin, Chem. Soc. Rev., 2010, 39, 1692–1705 RSC.
- D. A. Alonso and C. Najera, Chem. Soc. Rev., 2010, 39, 2891–2902 RSC; I. P. Beletskaya, A. N. Kashin, N. B. Karlstedt, A. V. Mitin, A. V. Cheprakov and G. M. Kazankov, J. Organomet. Chem., 2001, 622, 89–96 CrossRef CAS; V. P. W. Böhm and W. A. Herrmann, Chem. – Eur. J., 2001, 7, 4191–4197 CrossRef; J. E. Bollinger, O. Blacque and C. M. Frech, Chem. – Eur. J., 2008, 14, 7969–7977 CrossRef PubMed; M. R. Eberhard, Org. Lett., 2004, 6, 2125–2128 CrossRef PubMed; M. Nowotny, U. Hanefeld, H. van Koningsveld and T. Maschmeyer, Chem. Commun., 2000, 1877–1878 RSC; E. Peris, J. A. Loch, J. Mata and R. H. Crabtree, Chem. Commun., 2001, 201–202 RSC; G. Ren, X. Cui, E. Yang, F. Yang and Y. Wu, Tetrahedron, 2010, 66, 4022–4028 CrossRef; C. Rocaboy and J. A. Gladysz, New J. Chem., 2003, 27, 39–49 RSC; M. A. K. Vogel, C. B. W. Stark and I. M. Lyapkaloc, Adv. Synth. Catal., 2007, 349, 1019–1024 CrossRef.
- A. Avila-Sorrosa, F. Estudiante Negrete, S. Hernández Ortega, A. Toscano and D. Morales Morales, Inorg. Chim. Acta, 2010, 363, 1262–1268 CrossRef CAS; R. Gerber, O. Blacque and C. M. Frech, ChemCatChem, 2009, 1, 393 CrossRef; S. Sjovall, O. F. Wendt and C. Andersson, J. Chem. Soc., Dalton Trans., 2002, 1396–1400 RSC; Y. Ye, N. D. Ball, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2010, 132, 14682–14687 CrossRef PubMed.
- D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965–3972 CrossRef CAS PubMed.
- O. Blacque and C. M. Frech, Chem. – Eur. J., 2010, 16, 1521–1531 CrossRef CAS PubMed; A. Sundermann, O. Uzan and J. M. L. Martin, Chem. – Eur. J., 2001, 7, 1703–1711 CrossRef; Y. Dang, S. Qu, J. W. Nelson, H. D. Pham, Z.-X. Wang and X. Wang, J. Am. Chem. Soc., 2015, 137, 2006–2014 CrossRef PubMed.
- A. J. Martinez-Martinez, M. T. Chicote, D. Bautista and J. Vicente, Organometallics, 2012, 31, 3711–3719 CrossRef CAS.
- J. Vicente, A. Arcas, F. Julia-Hernandez and D. Bautista, Angew. Chem., Int. Ed. Engl., 2011, 50, 6896–6899 CrossRef CAS PubMed.
- F. Juliá-Hernández, A. Arcas and J. Vicente, Chem. – Eur. J., 2012, 18, 7780–7786 CrossRef PubMed.
- M.-J. Oliva-Madrid, J. A. García-López, I. Saura-Llamas, D. Bautista and J. Vicente, Organometallics, 2014, 33, 19–32 CrossRef CAS; A. Abellan-Lopez, M. T. Chicote, D. Bautista and J. Vicente, Dalton Trans., 2014, 43, 592–598 RSC; J. Vicente, J.-A. Abad, M.-J. Lopez-Saez, W. Förtsch and P. G. Jones, Organometallics, 2004, 23, 4414–4429 CrossRef.
- V. P. Boyarskiy, N. A. Bokach, K. V. Luzyanin and V. Y. Kukushkin, Chem. Rev., 2015, 115, 2698–2779 CrossRef CAS PubMed.
- J. J. Topczewski and M. S. Sanford, Chem. Sci., 2015, 6, 70–76 RSC; L. M. Xu, B. J. Li, Z. Yang and Z. J. Shi, Chem. Soc. Rev., 2010, 39, 712–733 RSC; P. Sehnal, R. J. K. Taylor and I. J. S. Fairlamb, Chem. Rev., 2010, 110, 824–889 CrossRef CAS PubMed; A. J. Canty, A. Ariafard, B. F. Yates and M. S. Sanford, Organometallics, 2015, 34, 1085–1090 CrossRef; C. P. Park, J. H. Lee, K. S. Yoo and K. W. Jung, Org. Lett., 2010, 12, 2450–2452 CrossRef PubMed; K. Muñiz, Angew. Chem., Int. Ed., 2009, 48, 9412–9423 CrossRef PubMed; N. M. Camasso, M. H. Perez-Temprano and M. S. Sanford, J. Am. Chem. Soc., 2014, 136, 12771–12775 CrossRef PubMed.
- T. Kesharwani, A. K. Verma, D. Emrich, J. A. Ward and R. C. Larock, Org. Lett., 2009, 11, 2591–2593 CrossRef CAS PubMed.
- A. J. Canty, M. C. Denney, B. W. Skelton and A. H. White, Organometallics, 2004, 23, 1122–1131 CrossRef CAS.
- J. Vicente, A. Arcas, F. Julia-Hernandez and D. Bautista, Chem. Commun., 2010, 46, 7253–7255 RSC.
- A. J. Canty, J. L. Hoare, N. W. Davies and P. R. Traill, Organometallics, 1998, 17, 2046–2051 CrossRef CAS; D. G. Brown, P. K. Byers and A. J. Canty, Organometallics, 1990, 9, 3080–3085 CrossRef; B. A. Markies, A. J. Canty, J. Boersma and G. van Koten, Organometallics, 1994, 13, 2053–2058 CrossRef.
- I. D. Rae, Can. J. Chem., 1966, 44, 1334 CrossRef CAS.
- F. Qu, J. R. Khusnutdinova, N. P. Rath and L. M. Mirica, Chem. Commun., 2014, 50, 3036–3039 RSC.
- J. Vicente, A. Arcas, F. Julia-Hernandez and D. Bautista, Inorg. Chem., 2011, 50, 5339–5341 CrossRef CAS PubMed.
- P. K. Byers, A. J. Canty, B. W. Skelton and A. H. White, Organometallics, 1990, 9, 826 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and spectroscopic data. CCDC 1414698. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc07698f |
| This journal is © The Royal Society of Chemistry 2016 |