Open Access Article
Open Access ArticleAnalytically useful blue chemiluminescence from a water-soluble iridium(III) complex containing a tetraethylene glycol functionalised triazolylpyridine ligand†
Zoe M.
Smith
a,
Emily
Kerr
a,
Egan H.
Doeven
b,
Timothy U.
Connell
ac,
Neil W.
Barnett
a,
Paul S.
Donnelly
c,
Stephen J.
Haswell
b and
Paul S.
Francis
*a
aCentre for Chemistry and Biotechnology, School of Life and Environmental Sciences, Deakin University, Locked Bag 20000, Geelong, Victoria 3220, Australia. E-mail: paul.francis@deakin.edu.au
bCentre for Regional and Rural Futures. Deakin University, Locked Bag 20000, Geelong, Victoria 3220, Australia
cSchool of Chemistry and Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, Melbourne, Victoria 3010, Australia
First published on 26th February 2016
Abstract
We examine [Ir(df-ppy)2(pt-TEG)]+ as the first highly water soluble, blue-luminescent iridium(III) complex for chemiluminescence detection. Marked differences in selectivity were observed between the new complex and the conventional [Ru(bpy)3]2+ reagent, which will enable this mode of detection to be extended to new areas of application.
Cyclometalated iridium(III) complexes exhibiting high luminescence efficiencies and a wide range of emission colours1 are seen as promising alternatives to the ruthenium(II) complexes traditionally employed for photoluminescence, chemiluminescence and electrochemiluminescence (ECL). The use of iridium(III) complexes offers opportunities to manipulate detection selectivity, shift the emission into the most sensitive wavelength range of conventional photomultiplier tubes, and develop multicolour detection systems.2,3 However, the poor aqueous solubility of many available iridium(III) complexes has limited their application in these areas. In cases where only low concentrations of the luminophore are required, such as photoluminescence protein staining, cellular imaging and molecular probes,4,5 or ECL labelling for immunoassay,6 sufficient solubility has been derived by including a neutral ligand to impart an overall positive charge to the complex, or a derivative containing sulfonate or saccharide groups.3,4,7–9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡ For chemiluminescence detection systems in which the metal complexes are used at relatively high concentrations to detect reducing agents (such as tertiary amines),10 these approaches have not resulted in adequate solubility and/or have restricted emission wavelengths to regions of green to red light. Nevertheless, explorations of iridium(III) complexes as chemiluminescence reagents7,9,11–13 have shown differences in the selectivity of their light producing reactions, and for some analytes, more intense emissions than those from analogous reactions with conventional ruthenium(II)-based reagents.7,9,12
‡ For chemiluminescence detection systems in which the metal complexes are used at relatively high concentrations to detect reducing agents (such as tertiary amines),10 these approaches have not resulted in adequate solubility and/or have restricted emission wavelengths to regions of green to red light. Nevertheless, explorations of iridium(III) complexes as chemiluminescence reagents7,9,11–13 have shown differences in the selectivity of their light producing reactions, and for some analytes, more intense emissions than those from analogous reactions with conventional ruthenium(II)-based reagents.7,9,12
We previously examined a series of chemiluminescence reactions of [Ir(C^N)2(BPS)]− complexes9,12 (where C^N represents the cyclometalating phenylisoquinoline (piq), phenylpyridine (ppy), phenylbenzothiazole (bt) or difluorophenylpyridine (df-ppy) ligands, and BPS is bathophenanthroline disulfonate), which exhibited red, orange, yellow and green emissions, respectively. The BPS ligand imparted significant solubility in aqueous solution, but a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water
1 mixture of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
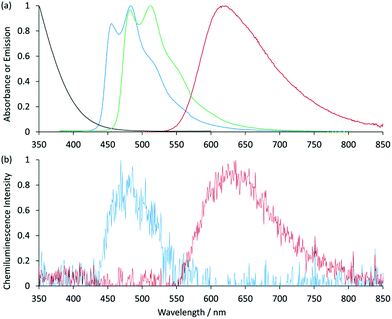
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile was required to prepare the reagents at 1 mM.9,12 Using a flow-injection analysis manifold, the reagents were oxidised with cerium(IV) sulfate before reacting with a variety of analytes. Greater chemiluminescence intensities were generally obtained using [Ir(df-ppy)2(BPS)]− or [Ir(bt)2(BPS)]− than with [Ru(bpy)3]2+, but the blank responses from the reaction between oxidised iridium complexes and solvent were also greater, which reduced the anticipated improvements in the signal-to-blank ratios.9,12 The inclusion of the BPS ligand in these complexes also induced a bathochromic shift in the emission when compared to their homoleptic counterparts, restricting the highest energy emission, exhibited by [Ir(df-ppy)2(BPS)]− (Fig. 1b), to the green region of the spectrum (λmax = 549 nm; Fig. S1 in ESI†),9 whereas the corresponding neutral [Ir(df-ppy)3] complex (Fig. 1a), which is not soluble in water, emits blue light (λmax = 495 nm).15
acetonitrile was required to prepare the reagents at 1 mM.9,12 Using a flow-injection analysis manifold, the reagents were oxidised with cerium(IV) sulfate before reacting with a variety of analytes. Greater chemiluminescence intensities were generally obtained using [Ir(df-ppy)2(BPS)]− or [Ir(bt)2(BPS)]− than with [Ru(bpy)3]2+, but the blank responses from the reaction between oxidised iridium complexes and solvent were also greater, which reduced the anticipated improvements in the signal-to-blank ratios.9,12 The inclusion of the BPS ligand in these complexes also induced a bathochromic shift in the emission when compared to their homoleptic counterparts, restricting the highest energy emission, exhibited by [Ir(df-ppy)2(BPS)]− (Fig. 1b), to the green region of the spectrum (λmax = 549 nm; Fig. S1 in ESI†),9 whereas the corresponding neutral [Ir(df-ppy)3] complex (Fig. 1a), which is not soluble in water, emits blue light (λmax = 495 nm).15
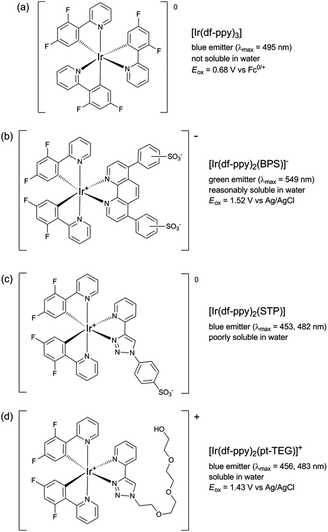 | ||
| Fig. 1 Comparison of [Ir(df-ppy)2(L)]n complexes, where L is: 2-(2,4-difluorophenyl)pyridinato (df-ppy);14,15 bathophenanthroline-disulfonate (BPS);9,12,16 1-phenylsulfonate-1,2,3-triazol-4-ylpyridine (STP);9 or 2-triethoxyethanol[4-(2-pyridinyl)-1H-1,2,3-triazole-1-yl] (pt-TEG).16,17 | ||
In a previous attempt to create a water-soluble iridium(III) complex exhibiting blue chemiluminescence,9 we synthesised a sulfonated derivative of 1-phenyl-1,2,3-triazol-4-ylpyridine (STP) as an alternative ancillary ligand to BPS. Although blue chemiluminescence was observed from [Ir(df-ppy)2(STP)] (Fig. 1c and S1†) upon reaction with cerium(IV) sulfate and pharmaceuticals such as codeine, furosemide and ofloxacin, the aqueous solubility of the complex was poor (limited to ∼10−5 M) and its chemiluminescence intensities (and signal/blank ratios) were generally very low compared to those of [Ru(bpy)3]2+ and the [Ir(C^N)2(BPS)]− complexes. However, a close analogue of [Ir(df-ppy)2(STP)] bearing a polyethylene glycol substituent ([Ir(df-ppy)2(pt-TEG)]+; Fig. 1d) was recently identified as a promising candidate for ECL detection,16 with high aqueous solubility and co-reactant ECL signals with tri-n-propylamine that were over twelve times greater than those of [Ru(bpy)3)]2+ when measured with a typical bialkali photomultiplier tube. Herein, we report our investigation of [Ir(df-ppy)2(pt-TEG)]+ as the first highly water-soluble iridium(III) complex exhibiting blue chemiluminescence.
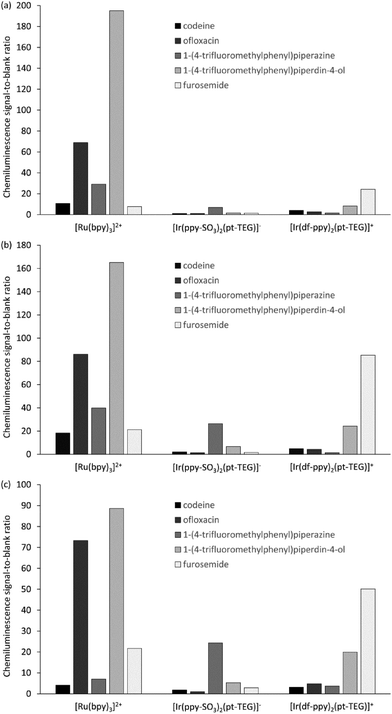
We compared the chemiluminescence responses of [Ir(df-ppy)2(pt-TEG)]+ with that of [Ru(bpy)3]2+ and a related iridium(III) complex that exhibits green luminescence ([Ir(ppy-SO3)2(pt-TEG)]−, Fig. 2a, S2 and S3†),§ using flow injection analysis methodology,¶ and reagent concentrations of 1 mM, 0.1 mM and 0.01 mM (Fig. 3), representing the wide range adopted in previous analytical applications.10 The compounds selected for the comparison: codeine, furosemide, 1-(4-trifluoromethylphenyl)piperazine, 1-(4-trifluoromethylphenyl)piperidin-4-ol and ofloxacin (Fig. S4†), have previously been shown to elicit light upon reaction with various ruthenium(II) and iridium(III) complexes under acidic conditions using cerium(IV) sulfate as an oxidant.9,18,19
Similar trends in the relative chemiluminescence intensities (and signal-to-blank ratios) were observed across the different reagent concentrations (Fig. 3 and S5†), but the three reagents exhibited markedly different selectivity. Using [Ru(bpy)3]2+, the greatest intensities were elicited by ofloxacin and the piperidinol derivative, whereas using [Ir(ppy-SO3)2(pt-TEG)]−, the piperazine derivative elicited a much greater response than the other four compounds. Lower signals (and S/B ratios) were observed using [Ir(df-ppy)2(pt-TEG)]+ than [Ru(bpy)3]2+, with the exception of the reaction with furosemide, which exhibited a four-fold greater S/B ratio with the blue-light emitting iridium(III) reagent (at 1 mM and 0.1 mM metal complex concentration).
The mechanism for the light-producing reactions of ruthenium- and iridium-complexes with various amine-containing compounds upon chemical or electrochemical oxidation involves numerous competing reaction pathways,20,21 the most dominant of which depends on the reaction conditions, and the properties of not only the metal complex, but also the amine and its radical oxidation products. The differences in the selectivity of the [Ru(bpy)3]2+, [Ir(ppy-SO3)2(pt-TEG)]− and [Ir(df-ppy)2(pt-TEG)]+ complexes towards these analytes can be attributed to factors such as their oxidising strength (Eox = 1.06 V, 1.09 V and 1.43 V vs. Ag/AgCl, respectively16,20), ligand structure and overall charge (2+, 1− and 1+, respectively), which influence the rate of reaction leading to the emitting species. Similar reasoning can be made for the deleterious light-producing reaction with the solvent that produces the ‘blank’ response. The chemiluminescence intensity of the three complexes with any particular analyte (or the solvent) will also be limited by the luminescence quantum yield of each complex.
The optimum reagent concentration, in terms of chemiluminescence S/B ratios, was found to be both analyte and reagent dependent, in agreement with our previous investigations.12 In that prior work, stopped-flow experiments indicated that the changes in S/B ratio arose from the influence of concentration on the rates of the competing reactions of analyte and solvent with the reagent, coupled with the dependence of the chemiluminescence signal measured in a flow-injection analysis system on the reaction kinetics.12
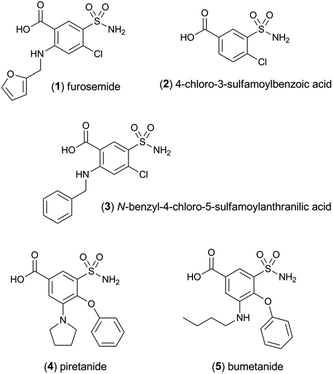
The sensitivity of the [Ir(df-ppy)2(pt-TEG)]+ reagent towards furosemide compared to the other analytes under investigation is similar under certain conditions to that observed for [Ir(df-ppy)2(BPS)]− (Fig. 1b),12 which also has a much higher Eox (1.52 V vs. Ag/AgCl)16 than [Ru(bpy)3]2+. Comparison of the chemiluminescence intensity of [Ru(bpy)3]2+ and [Ir(df-ppy)2(pt-TEG)]+ with compounds similar in structure to furosemide (Fig. 4 and 5) highlighted the remarkable difference in selectivity between the two reagents. Removing the aniline group from furosemide, or replacing its furan-2-ylmethyl substituent with a benzyl group on the anilinic nitrogen reduced the chemiluminescence intensity with [Ir(df-ppy)2(pt-TEG)]+ by an order of magnitude (Fig. 6). Piretanide, which possesses a tertiary aniline group, gave significantly greater chemiluminescence intensity upon reaction with [Ru(bpy)3]2+ (and cerium(IV)) than the other compounds, but this was not observed for [Ir(df-ppy)2(pt-TEG)]+.
 | ||
| Fig. 4 Photographs of the chemiluminescence reactions of (a) [Ru(bpy)3]2+ and 1-(4-trifluoromethylphenyl)piperdin-4-ol, (b) [Ir(ppy-SO3)2(pt-TEG)]− and 1-(4-trifluoromethylphenyl)piperazine, and (c) [Ir(df-ppy)2(pt-TEG)]+ and furosemide, in aqueous solution. The metal complex reagents were continuously merged with an oxidant solution (cerium(IV) sulfate in 0.05 M H2SO4) at a T-piece shortly prior to mixing with the other reactant solution within a transparent serpentine flow-cell.22 A Canon 6D camera with 50 mm f1.8 lens were used (Canon, Japan). The exposure time and reactant concentrations were adjusted to produce similar emission intensities. | ||
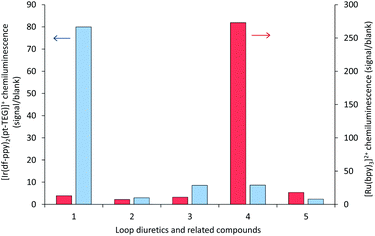 | ||
| Fig. 6 Chemiluminescence responses (signal/blank ratios) of various loop diuretics and related compounds: (1) furosemide, (2) 4-chloro-3-sulfamoyl-benzoic acid, (3) N-benzyl-4-chloro-sulfamoylanthranillic acid, (4) piretanide, (5) bumetanide at 1 μM, with [Ru(bpy)3]2+ (red columns) and [Ir(df-ppy)2(pt-TEG)]+ (blue columns), using flow injection analysis methodology. Reagent concentration: 0.1 mM. Oxidant: 1 mM cerium(IV) sulfate in 0.05 M H2SO4. A comparison of signal/blank ratios for the two complexes with the same y-axis scale is shown in Fig. S6 in the ESI.† | ||
Previously examined iridium(III) complexes often exhibited higher chemiluminescence intensities than [Ru(bpy)3]2+,9,12 but also showed greater blank responses from the corresponding reaction with the solvent. However, this was not the case for [Ir(df-ppy)2(pt-TEG)]+, which gave lower blank responses than [Ru(bpy)3]2+. We suspected that this might be due to partial absorption of the blue emission of [Ir(df-ppy)2(pt-TEG)]+ by the cerium(IV) oxidant, but the overlap of their emission and absorption spectra is minimal (Fig. 2a).|| Consequently, there was no red-shift in the chemiluminescence emission compared to the photoluminescence in the absence of cerium(IV) (Fig. 2b): visually, the light emitted from the reaction of [Ir(df-ppy)2(pt-TEG)]+ with cerium(IV) sulfate and furosemide (Fig. 4) was the same blue colour as the corresponding photoluminescence (Fig. S3†).
Calibrations for furosemide (Fig. S7†) prepared using flow injection analysis methodology¶ with 0.1 mM [Ir(df-ppy)2(pt-TEG)]+ (and 1 mM cerium(IV) sulfate in 0.05 mM H2SO4) showed a superior limit of detection (1 × 10−8 M; 3σ) than that obtained with [Ru(bpy)3]2+ (7 × 10−8 M; 3σ) under the same conditions. These detection limits are comparable to those reported in previous studies based on chemiluminescence reactions with [Ru(bpy)3]2+ (8 × 10−9 M to 2 × 10−7 M),12,23,24 [Ru(BPS)3]4− (1 × 10−8 M to 3 × 10−8 M),18,24 and [Ir(df-ppy)2(BPS))]− (1 × 10−8 M),12 where cerium(IV) was used as the oxidant.
Conclusions
These preliminary investigations of the [Ir(df-ppy)2(pt-TEG)]+ complex as a chemiluminescence reagent reveal a viable approach to develop new detection systems based on chemically induced blue luminescence from metal complexes under analytically useful aqueous conditions. The use of polyethylene glycol groups is a more effective option to enhance solubility in water than previous attempts involving ligands with sulfonate groups.9,12 The ability to shift the emission bands into the blue region of the visible spectrum is advantageous for the development of miniaturised analytical devices with low-cost photodetectors. Moreover, the striking differences in the selectivity of these novel chemiluminescence reagents compared to traditional ruthenium(II) polypyridine complexes will expand the scope of chemiluminescence detection into new areas of application.Acknowledgements
We thank the Australian Research Council (DP140100439, FT100100646) for funding this research.Notes and references
- M. S. Lowry and S. Bernhard, Chem. – Eur. J., 2006, 12, 7970–7977 CrossRef CAS PubMed; L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura and F. Barigelletti, Top. Curr. Chem., 2007, 281, 143–203 CrossRef; Y. You and W. Nam, Chem. Soc. Rev., 2012, 41, 7061–7084 RSC.
- A. Kapturkiewicz and G. Angulo, Dalton Trans., 2003, 3907–3913 RSC; P. Coppo, E. A. Plummer and C. L. De, Chem. Commun., 2004, 1774–1775 RSC; J. I. Kim, I.-S. Shin, H. Kim and J.-K. Lee, J. Am. Chem. Soc., 2005, 127, 1614–1615 CrossRef CAS PubMed; K. N. Swanick, S. Ladouceur, E. Zysman-Colman and Z. Ding, Chem. Commun., 2012, 48, 3179–3181 RSC; E. H. Doeven, E. M. Zammit, G. J. Barbante, C. F. Hogan, N. W. Barnett and P. S. Francis, Angew. Chem., Int. Ed., 2012, 51, 4354–4357 CrossRef PubMed; E. H. Doeven, G. J. Barbante, E. Kerr, C. F. Hogan, J. A. Endler and P. S. Francis, Anal. Chem., 2014, 86, 2727–2732 CrossRef PubMed.
- S. Zanarini, M. Felici, G. Valenti, M. Marcaccio, L. Prodi, S. Bonacchi, P. Contreras-Carballada, R. M. Williams, M. C. Feiters, R. J. M. Nolte, L. De Cola and F. Paolucci, Chem. – Eur. J., 2011, 17, 4640–4647 CrossRef CAS PubMed.
- J. Jia, H. Fei and M. Zhou, Electrophoresis, 2012, 33, 1397–1401 CrossRef CAS PubMed; Q. Zhao, M. Yu, L. Shi, S. Liu, C. Li, M. Shi, Z. Zhou, C. Huang and F. Li, Organometallics, 2010, 29, 1085–1091 CrossRef.
- S. Lin, W. Gao, Z. Tian, C. Yang, L. Lu, J.-L. Mergny, C.-H. Leung and D.-L. Ma, Chem. Sci., 2015, 6, 4284–4290 RSC.
- X. Zhou, D. Zhu, Y. Liao, W. Liu, H. Liu, Z. Ma and D. Xing, Nat. Protoc., 2014, 9, 1146–1159 CrossRef CAS PubMed; K. Muzyka, Biosens. Bioelectron., 2014, 54, 393–407 CrossRef PubMed.
- R. V. Kiran, E. M. Zammit, C. F. Hogan, B. D. James, N. W. Barnett and P. S. Francis, Analyst, 2009, 134, 1297–1298 RSC.
- M. Felici, P. Contreras-Carballada, Y. Vida, J. M. Smits, R. J. Nolte, L. De Cola, R. M. Williams and M. C. Feiters, Chem. – Eur. J., 2009, 15, 13124–13134 CrossRef CAS PubMed; M.-J. Li, P. Jiao, W. He, C. Yi, C.-W. Li, X. Chen, G.-N. Chen and M. Yang, Eur. J. Inorg. Chem., 2011, 197–200 CrossRef.
- J. Truong, K. B. Spilstead, G. J. Barbante, E. H. Doeven, D. J. D. Wilson, N. W. Barnett, L. C. Henderson, J. M. Altimari, S. C. Hockey, M. Zhou and P. S. Francis, Analyst, 2014, 139, 6028–6035 RSC.
- B. A. Gorman, P. S. Francis and N. W. Barnett, Analyst, 2006, 131, 616–639 RSC.
- J. M. Terry, E. M. Zammit, T. Slezak, N. W. Barnett, D. C. Olson, D. K. Wolcott, D. L. Edwards and P. S. Francis, Analyst, 2011, 136, 913–919 RSC.
- E. M. Zammit, N. W. Barnett, L. C. Henderson, G. A. Dyson, M. Zhou and P. S. Francis, Analyst, 2011, 136, 3069–3072 RSC.
- F. Wu, B. Tong and Q. Zhang, Anal. Sci., 2011, 27, 529–533 CrossRef CAS PubMed; F. Wu, B. Tong, X. Wei and L. Chen, Luminescence, 2012, 27, 519–523 CrossRef PubMed; Y. P. Dong, L. Huang, B. H. Tong, M. J. Shi, W. B. Zhang and Q. F. Zhang, Luminescence, 2012, 27, 262–267 CrossRef PubMed; Y. P. Dong, M. J. Shi, B. H. Tong and Q. F. Zhang, Luminescence, 2012, 27, 414–418 CrossRef PubMed.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, P. S. Francis, N. W. Barnett and C. F. Hogan, Chem. Sci., 2013, 4, 977–982 RSC.
- G. J. Barbante, E. H. Doeven, E. Kerr, T. U. Connell, P. S. Donnelly, J. M. White, T. Lópes, S. Laird, C. F. Hogan, D. J. D. Wilson, P. J. Barnard and P. S. Francis, Chem. – Eur. J., 2014, 20, 3322–3332 CrossRef CAS PubMed.
- E. Kerr, E. H. Doeven, G. J. Barbante, T. U. Connell, P. S. Donnelly, D. J. D. Wilson, T. D. Ashton, F. M. Pfeffer and P. S. Francis, Chem. – Eur. J., 2015, 21, 14987–14995 CrossRef CAS PubMed.
- E. H. Doeven, G. J. Barbante, A. J. Harsant, P. S. Donnelly, T. U. Connell, C. F. Hogan and P. S. Francis, Sens. Actuators, B, 2015, 216, 608–613 CrossRef CAS.
- J. Xi, X. Ji, S. Zhang, X. Ai and Z. He, Anal. Chim. Acta, 2005, 541, 193–198 CrossRef CAS.
- L. Chen, X. Wang, H. Zhao, K. Wang and L. Jin, Luminescence, 2008, 23, 309–315 CrossRef CAS PubMed; P. S. Francis and J. L. Adcock, Anal. Chim. Acta, 2005, 541, 3–12 CrossRef; B. Rezaei, T. Khayamian and A. Mokhtari, J. Pharm. Biomed. Anal., 2009, 49, 234–239 CrossRef PubMed; R. J. Waite, G. J. Barbante, N. W. Barnett, E. M. Zammit and P. S. Francis, Talanta, 2013, 116, 1067–1072 CrossRef PubMed.
- W. Miao, J.-P. Choi and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 14478–14485 CrossRef CAS PubMed.
- C. M. Hindson, G. R. Hanson, P. S. Francis, J. L. Adcock and N. W. Barnett, Chem. – Eur. J., 2011, 17, 8018–8022 CrossRef CAS PubMed; E. Kerr, E. H. Doeven, D. J. D. Wilson, C. F. Hogan and P. S. Francis, Analyst, 2016, 141, 62–69 RSC.
- S. Mohr, J. M. Terry, J. L. Adcock, P. R. Fielden, N. J. Goddard, N. W. Barnett, D. K. Wolcott and P. S. Francis, Analyst, 2009, 134, 2233–2238 RSC.
- F. Maya, J. M. Estela and V. Cerdà, Talanta, 2010, 80, 1333–1340 CrossRef CAS PubMed.
- G. P. McDermott, E. M. Zammit, E. K. Bowen, M. M. Cooke, J. L. Adcock, X. A. Conlan, F. M. Pfeffer, N. W. Barnett, G. A. Dyson and P. S. Francis, Anal. Chim. Acta, 2009, 634, 222–227 CrossRef CAS PubMed.
- H. Horváth, Á. Kathó, A. Udvardy, G. Papp, D. Szikszai and F. Joó, Organometallics, 2014, 33, 6330–6340 CrossRef; H. Horváth, G. Papp, R. Szabolcsi, Á. Kathó and F. Joó, ChemSusChem, 2015, 8, 3036–3038 CrossRef PubMed.
- J. M. Terry, J. L. Adcock, D. C. Olson, D. K. Wolcott, C. Schwanger, L. A. Hill, N. W. Barnett and P. S. Francis, Anal. Chem., 2008, 80, 9817–9821 CrossRef CAS PubMed.
- P. S. Francis, J. L. Adcock and N. W. Barnett, Spectrochim. Acta, Part A, 2006, 65, 708–710 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemical structure of [Ir(ppy-SO3)2(pt-TEG)]−, photograph of the luminescence, structure of compounds selected for the comparison, additional relative chemiluminescence responses, additional spectroscopic data, and calibration graphs. See DOI: 10.1039/c6an00141f |
| ‡ Similarly, water-soluble iridium-based catalysts have been prepared incorporating a tertiary phosphine ligand with multiple sulfonate substituents.25 |
| § [Ir(df-ppy)2(pt-TEG)]Cl and Na[Ir(ppy-SO3)2(pt-TEG)] were prepared as previously described.16 [Ru(bpy)3]Cl2·6H2O was purchased from Strem (MA, USA). |
| ¶ Flow injection analysis was used to reproducibly combine the reactants. The manifold was assembled as described previously,11 which included a GloCel chemiluminescence detector (Global FIA, MA, USA) with dual-inlet serpentine flow-cell26 and an Electron Tubes model 9125B photomultiplier tube (ETP, NSW, Australia). The aqueous metal-complex reagent solutions were injected (70 μL) into a carrier line containing the 1 mM cerium(IV) sulfate (in 0.05 M H2SO4), which merged with the analyte solution within the detection flow-cell. A flow rate of 3.5 mL min−1 per line was used in all experiments. |
| || Absorption spectra were obtained using Cary 300 Spectrophotometer. Photoluminescence spectra were obtained with a Cary Eclipse fluorescence spectrophotometer (5 nm excitation and emission band pass) and corrected for the wavelength dependence of the detector response and monochromator transmission.27 Chemiluminescence spectra were obtained by replacing the photomultiplier tube in the flow injection analysis manifold with an Ocean Optics QE65Pro spectrometer with CCD detector (10 s spectra integration time, each acquisition manually triggered in concert with reagent injection), which was interfaced with the chemiluminescence flow-cell via fibre optic cable (1 mm core diameter, 1.0 m length) and collimating lens (30 mm diameter, 350–2000 nm). This enabled measurement of the chemiluminescence spectrum after each injection, as the light-producing reaction mixture passed through the flow-cell. The presented chemiluminescence spectra are each an average of those obtained from three injections of the reagent solution into the flow injection analysis manifold. |
| This journal is © The Royal Society of Chemistry 2016 |