Fabrication of silver-decorated sulfonated polystyrene microspheres for surface-enhanced Raman scattering and antibacterial applications
Abstract
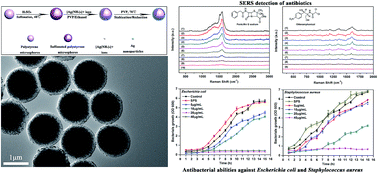
A facile approach was proposed to decorate silver nanoparticles on sulfonated polystyrene microspheres (denoted as SPS@Ag composite microspheres), in which polyvinylpyrrolidone (PVP) served both as the reductant and stabilizer. In this approach, first, sulfonated polystyrene (SPS) microspheres were synthesized via the sulfonation of monodisperse polystyrene (PS) microspheres in the concentrated sulfuric acid, which were used as the active template cores. Upon the electrostatic attraction between the negatively charged –SO3H groups and the positively charged [Ag(NH3)2]+ ions, silver precursor-[Ag(NH3)2]+ ions were easily adsorbed onto the surfaces of sulfonated polystyrene microspheres. These [Ag(NH3)2]+ ions were in situ reduced to metallic Ag nanoparticles and simultaneously protected by PVP, forming the stable SPS@Ag composite microspheres. During the reaction, neither additional reducing agents nor stabilizers were needed. Moreover, by simply adjusting the concentration of [Ag(NH3)2]+ ions, the size of Ag nanoparticles and the surface coverage of SPS with Ag nanoparticles can be easily tailored. Finally, it has been proven that these as-synthesized SPS@Ag composite microspheres were the ideal active substrates for the surface-enhanced Raman spectroscopy (SERS) for the trace detection trace of the antibiotics (i.e., penicillin G sodium and chloramphenicol), and showed an enhanced antibacterial activity against both Escherichia coli (Gram-negative bacteria) and Staphylococcus aureus (Gram-positive bacteria) as well.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...