Selective synthesis of different ZnO/Ag nanocomposites as surface enhanced Raman scattering substrates and highly efficient photocatalytic catalysts
Abstract
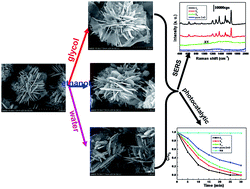
ZnO/Ag nanocomposites with adjustable surface-enhanced Raman scattering performance and photocatalytic activity were synthesized by a photochemical method. The as-prepared samples were investigated using various spectroscopic and microscopic techniques in detail. Solvents play a key role in controlling the morphologies and properties of these nanocomposites. Detection and degradation of organic dyes is studied. It has been found that suitable experimental parameters are crucial to the detection and degradation of organic dyes molecules. The work is of great importance in practical applications based on the surface-enhanced Raman scattering and photocatalytic performance of noble metal/oxide metal nanocomposites, which could be used for detection and degradation of organic dyes and pollutants.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances


 Please wait while we load your content...
Please wait while we load your content...