Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Usage of different vessel models in a flow-through cell: in vitro study of a novel coated balloon catheter
Jenny
Bandomir
a,
Sebastian
Kaule
b,
Klaus-Peter
Schmitz
b,
Katrin
Sternberg
b,
Svea
Petersen
b and
Udo
Kragl
*a
aUniversity of Rostock, Department of Chemistry, Albert-Einstein-Straße 3a, 18059 Rostock, Germany. E-mail: udo.kragl@uni-rostock.de
bUniversity of Rostock, Institute for Biomedical Engineering, Friedrich-Barnewitz-Straße 4, 18119 Rostock, Germany
First published on 9th January 2015
Abstract
Drug-coated balloon catheters are a novel clinical treatment alternative for coronary and peripheral artery diseases. Calcium alginate, poly(vinylethylimidazolium bromide) and polyacrylamide hydrogels were used as vessel models in this in vitro study. In comparison to a simple silicone tube their properties can be easily modified simulating different types of tissue. Local drug delivery after balloon dilation in the first crucial minute was determined in a vessel-simulating flow-through cell by a simulated blood stream. Balloon catheters were coated with paclitaxel using the ionic liquid cetylpyridinium salicylate as a novel carrier. Drug transfer from coated balloon catheters to different simulated vessel walls was evaluated and compared to a silicone tube. The highest paclitaxel delivery upon dilation was achieved with calcium alginate as the vessel model (60%) compared to polyacrylamide with 20% drug transfer. The silicone tube showed the least amount of wash-off (<1%) by a simulated blood stream after one minute from the vessel wall. The vessel-simulating flow-through cell was combined with a model coronary artery pathway to estimate drug loss during simulated use in an in vitro model. Calcium alginate and polyacrylamide hydrogels were used as tissue models for the simulated anatomic implantation process. In both cases, similar transfer rates for paclitaxel upon dilation were detected.
1. Introduction
Drug-coated balloon (DCB) technologies have emerged as a potential alternative to drug eluting stents (DES) to minimize restenosis.1 The applied drug should exhibit specific chemical properties and mechanism of action as well as pharmacokinetics and a fast transfer to be quickly absorbed by the vessel wall.2 Paclitaxel (PTX), a cytotoxic agent, was determined as the primary drug for DCB due to its efficient uptake as well as its extended retention.3 The cytotoxic, anti-proliferative effect of DES on the vessel wall has been widely explored.2,4 Preclinical studies with DCB have shown that 3 μg mm−2 paclitaxel is the effective dose to achieve an efficient, long-term, antiproliferative effect on the vessel wall.2,5 Drug delivery during angioplasty depends on drug dose, transfer system, dilation time, release pattern and appropriate balloon coating. Different balloon coating technologies are described in the literature.2,3,6–8 In addition to pure PTX balloon catheters different PTX formulations with additives such as urea, butyryl-tri-hexyl citrate (BTHC), iopromide and Shellac (aleuritic and shellolic acid) are commercially available.6 Kleber et al. summarized clinical evidence for these different DCBs in coronaries arteries with CE-mark (Conformité Européene).7The Paccocath technology with PTX embedded in hydrophilic iopromide coating increases the solubility and thus the transfer of PTX to the vessel wall. More than 80% of the drug is retained during balloon implantation to the target tissue (lesion) and 10–15% of the drug is released in the vessel wall upon 60 s balloon inflation.3 FreePac technology uses the natural additive urea as a carrier, which should enhance drug release as well as absorption, and thereby reduce total drug elution times (30–60 s). During balloon inflation, the blood flow in the vessel is interrupted and therefore expansion can only be maintained up to one minute. Microporous balloon surfaces with Shellac coating technology can be inflated up to one minute and achieve total drug release. A shorter dilation time results in partial drug release.2,3 In a porcine model vessel wall, Scheller et al. have demonstrated a drug release of approx. 90% after one minute inflation and 40 to 60 minutes later, they could detect about 10% PTX in the vessel wall. Thus, PTX is transferred into and retained by the pig tissue for a certain time.9
To date, there are only a few in vitro studies presented, characterizing and describing the simulated use of drug coated balloon catheters in an in vitro vessel model.10–12 The previously used models are far from physiological properties of the material, e.g. a silicone tube acts like an artery. We have been working on polymerized ionic liquids (PILs) which are able to form hydrogels. Depending on the type of ionic liquid and degree of cross-linking the mechanical properties can be modified.13 In our presented study, these hydrogels were evaluated to act as vessel model and compared to known hydrogels. Next to calcium alginate as a natural hydrogel, synthetic polymers with good mechanical and long-term stability were also used.13 PTX-coated balloon catheters using the ionic liquid cetylpyridinium salicylate (Cetpyrsal) as a novel innovative additive were studied.11 Local drug delivery within a vessel-simulating flow-through cell under physiological conditions during the first crucial minute was investigated. For an assessment of this study, the total drug delivery upon dilation (retention into the hydrogel and wash-off (release) from the hydrogel compartment by a simulated blood stream) and the residual load on the balloon were analyzed. Furthermore, the drug loss during a simulated insertion was estimated by combining the flow-through cell with a model coronary artery pathway.
2. Materials and methods
2.1. Materials
Paclitaxel (PTX, ≥99.5%) was obtained from Cfm Oskar Tropitzsch e.K., Germany. Sodium alginate was purchased from Fagron GmbH & Co KG, Germany. Calcium sulfate dihydrate (≥99%; Merck KGaA, Germany) and trisodium phosphate decahydrate (≥99%; Merck KGaA, Germany) were used as received. 1-Vinylimidazole (≥99%; Sigma-Aldrich, Germany), ethyl bromide (≥99%; Merck KGaA, Germany), cetylpyridinium chloride (Cetpyr, ≥96%; AppliChem GmbH, Germany) and sodium salicylate (≥99.5%; Merck KGaA, Germany) were also used as received. Ammonium peroxydisulphate (APS, ≥98%), tetramethylethylenediamine (TEMED, 99%), Rotiphorese® Gel 30 (acrylamide/bisacrylamide, 37.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Rotiphorese® Gel B (2% bisacrylamide) were obtained from Carl Roth, Germany and used as received. Angioplasty balloon catheters of 4 mm in diameter and 20 or 30 mm length were kindly supplied by Biotronik SE & Co KG, Germany. Ionic liquid (IL) cetylpyridinium salicylate (Cetpyrsal) was synthesized by reaction of cetylpyridinium chloride with sodium salicylate according to published procedures.11,14
1) and Rotiphorese® Gel B (2% bisacrylamide) were obtained from Carl Roth, Germany and used as received. Angioplasty balloon catheters of 4 mm in diameter and 20 or 30 mm length were kindly supplied by Biotronik SE & Co KG, Germany. Ionic liquid (IL) cetylpyridinium salicylate (Cetpyrsal) was synthesized by reaction of cetylpyridinium chloride with sodium salicylate according to published procedures.11,14
2.2. Balloon coating
A pipetting technique was used for the coating of the inflated balloon catheter according to Petersen et al.11 Briefly, PTX and Cetpyrsal were separately dissolved in methanol to yield concentrations of 4.72 mg mL−1 (both stock solutions). Following this, a Cetpyrsal–PTX solution (50%, w/w) was mixed from both stock solutions. 100 μL of the Cetpyrsal–PTX solution was then slowly pipetted onto each balloon catheter, resulting in a PTX surface load of approx. 3 μg mm−2, respectively a total of 659.73 μg (balloon 1: 3.5 mm diameter, 20 mm length), 753.99 μg (balloon 2: 4.0 mm diameter, 20 mm length) or 1130.97 μg (balloon 3: 4.0 mm diameter, 30 mm length). During the pipetting process, the balloon was rotated and evaporation of methanol was ensured by a gentle stream of air. Finally, all balloon catheters were dried at 23 ± 2 °C overnight.112.3. Hydrogel preparation
2.4. Simulated use of DCB in a flow-through cell in different vessel models
An adapted vessel-simulating flow-through cell was chosen, which is described in detail by Seidlitz et al.15 Instead of the acrylic glass disc a metal disc was used. Calcium alginate, PAAm and poly(VEImBr) hydrogels were inserted as hydrogel compartments. The DCB was placed in the simulated vessel wall and dilated for 60 s with a nominal pressure of 7 bar. The flow-through cell with the inflated balloon catheter is shown in Fig. 1 with PAAm hydrogel as the vessel model. After expansion, the balloon was removed and isotonic sodium chloride (NaCl, 0.9%) as a perfusion medium was circulated along the simulated vessel wall for a duration of 1 min at a flow rate of 35 mL min−1. Pumping of medium was managed with a gear pump (Ismatec MCP-Z ISM 405A, pump head model 186-000, Germany; Tygon® tube R 3607, 3.17 mm ID, VWR International GmbH, Germany) and the set flow rate was adjusted to the blood flow velocity in coronaries.16 The isotonic solution was collected in a falcon vessel and the PTX concentrations were measured by HPLC (see HPLC parameters). The process of balloon angioplasty was simulated applying an in vitro model, consisting of a guiding catheter (Cordis® Vista Brite Tip®; 6F; 1.75 mm ID; 90 cm) with a guide wire (Biotronik SE & Co KG, Galeo M 014) and a flow-through cell with different hydrogel compartments at the end of the test path, representing the vessel wall. Experiments were also performed with a silicone tube (3.0 mm ID) as the vessel model to compare the results. Paclitaxel transfer into different simulated vessel walls was measured. Before balloon dilation, the guiding catheter and vessel model were flushed with 20 mL NaCl-solution (0.9%). PTX-coated balloon catheters using Cetpyrsal additive were inserted into the guiding catheter and via a guide wire, the balloon catheter was placed in the simulated vessel wall.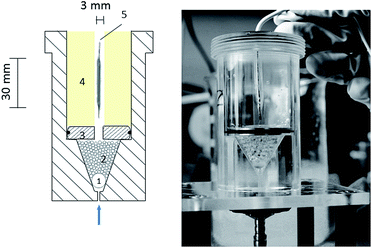 | ||
| Fig. 1 Schematic drawing of the flow through cell; 1 large glass bead, 2 small glass beads, 3 metal disc, 4 hydrogel matrix, 5 drug eluting ballon (DEB). | ||
After balloon deflation, the pump was started (flow rate 35 mL min−1). The PTX concentration simulating drug wash-off from the vessel model within the first crucial minute was determined by HPLC measurements. The guiding catheter was then flushed with 20 mL methanol. The balloon catheter was extracted in 10 mL methanol for 30 min at 23 ± 2 °C and then the residue on the balloon was analyzed. The used hydrogel after cutting into small pieces was also extracted with methanol (20 mL) for 30 min at 23 ± 2 °C to detect the amount of transferred drug into the vessel model. The entire guiding catheter was then flushed with 20 mL of 0.9% NaCl-solution in preparation of the next experiment. In summary, the total PTX delivery upon dilation composed of drug transfer into the hydrogel and drug wash-off from the hydrogel compartment after 1 min by a simulated blood stream. All samples were quantified by means of HPLC after a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution with methanol.
2 dilution with methanol.
2.5. Simulated use of DCB in the vessel-simulating flow-through cell after passage through an in vitro vessel model according to ASTM F2394-07
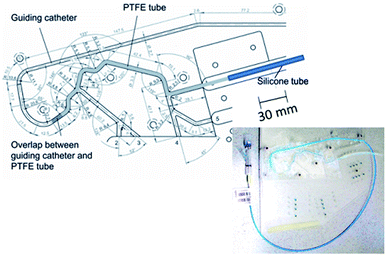
A standard anatomic model adapted from ASTM F2394-07, recently described in the literature as a standard procedure, was applied to simulate the implantation process of DCB.17 The model consisted of polymethacrylate plates forming a simulated course of a coronary artery. The used guiding catheter (Cordis® Vista Brite Tip®; 6F; 1.75 mm ID; 90 cm) with a guide wire (Biotronik SE & Co KG, Galeo M 014) and the tortuous path equipped with a PTFE tube was placed in a 37 ± 2 °C heated water bath (Fig. 2). The model was flushed with 30 mL 0.9% NaCl-solution. A DCB was introduced into the guiding catheter of the model and initially placed at the end of the PTFE tube. The guiding catheter was then flushed with 30 mL 0.9% NaCl-solution to recover particles and PTX released during tracking. At the distal end of the test path, a hydrogel vessel model (calcium alginate or PAAm) was placed and the balloon was dilated to 7 bar and held for 1 min. The balloon was removed after deflation and extracted in 20 mL methanol for 10 min (residual PTX load on the balloon) at 23 ± 2 °C. The pump was then started (flow rate 35 mL min−1) and the PTX concentration simulating the drug wash-off in the first crucial minute was determined. Then the used hydrogel after cutting was also extracted with methanol (20 mL) for 30 min at 23 ± 2 °C (drug transfer into the vessel model). After balloon extraction (10 min) in methanol, the balloon was removed and the entire pathway was then finally flushed with 30 mL methanol. Subsequently, the test path was flushed with 0.9% NaCl-solution in preparation of the next balloon dilation.The total PTX delivery upon dilation composed of drug transfer into the vessel model (hydrogel) and drug wash-off from the hydrogel compartment after 1 min by a simulated blood stream. All samples were quantified by means of HPLC after a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution with methanol.
2 dilution with methanol.
3. Results and discussion
3.1. Comparison of different hydrogels in the flow-through cell
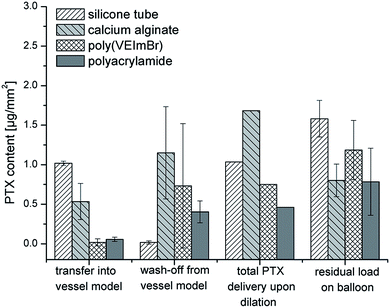
The first set of experiments of DCB compared different hydrogels as tissue models to evaluate drug release of PTX. Drug transfer, the retention of PTX into three different hydrogels as tissue models respectively vessel walls as well as the wash-off (release) from the hydrogel compartment within a vessel-simulating flow-through cell were investigated during balloon dilation. A PTX transfer should be examined by using different hydrogel compartments to determine the influence of the tissue model relating to the PTX transfer upon dilation. Certain properties of the used hydrogels to simulate a vessel wall such as permeability, flexibility and long-term stability of synthetic polymers (poly(VEImBr) and PAAm) are of particular importance. Calcium alginate as a natural polymer is easily accessible but has limited long-term stability. Monovalent cations such as Na+ dissolve the network within short time. In addition, alginate hydrogels are prone to microbial contamination. Results for various vessel models are depicted in Fig. 3. The total PTX delivery upon dilation composed of drug transfer into the hydrogel and drug wash-off from the hydrogel compartment after 1 min by a simulated blood stream. In the following the results from the balloon dilations will be discussed.| Wash-off after 1 min | Transfer into vessel model | Total PTX delivery upon delivery | |
|---|---|---|---|
| a n. a.: not available. | |||
| Silicone tube | <1% | 38.6 ± 3.4% | About 40% |
| Calcium alginate | 41.2 ± 14.2% | 21.4 ± 10.7% | About 60% |
| Poly(VEImBr) | 28.7 ± 26.2% | <2% | n. a. |
| PAAm | 17.8 ± 5.3% | 2.8 ± 1.8% | About 20% |
Furthermore, the hydrogel characteristics were important for PTX transfer and diffusion into hydrogels.21 PAAm and poly(VEImBr) were synthetic polymers with a specific cross-linker content (poly(VEImBr): 1.7% to PAAm: 0.8% cross-linker content).13 On the contrary, the calcium alginate hydrogel is a natural polymer with variability in its properties. In addition to mechanical properties (flexibility) of the vessel models, different adhesion properties were present. This corresponds to different amounts of PTX wash-off from the vessel models after 1 min by a simulated blood stream (see Table 1 or Fig. 3). Moreover, the diffusion of PTX into the vessel wall occurs at various rates, which may be related with the cross-linker content. This leads to PTX diffusion into synthetic polymers < 5% (poly(VEImBr) and PAAm) compared to the natural polymer of 21.4 ± 10.7%.
As already mentioned, PTX is characterized by its very low solubility. The balloon catheters used here exhibit homogeneous coating due to the use of an IL as a novel additive (Cetpyrsal/PTX, 50/50, w/w). There are no needle-like crystals present on the balloon surface.11 Previous experiments showed that the novel additive reduced the drug loss compared to a commercially available DCB with an urea-based coating.11 For this reason, there is the possibility to deliver (transfer) more PTX during the balloon expansion and therefore we concentrated on this novel DCB. The degree of crystallization is important; Afari et al. published that more crystalline coatings yield higher tissue levels and biological efficacy.25 In contrast, less crystalline coatings resulted in improved uniformity and less particle formation.25 Heilmann et al. had found (via an in vivo study) that the advantageous effect of a hydrophilic additive such as using iopromide for higher tissue concentrations was antagonized by increased amounts of wash-off of used coatings.26
Drug loss is a process constituted of mechanical loss by sheath passage and collisions with the vessel wall and dissolution of the coating in the blood stream.26 This process will be simulated using a standard anatomic model adapted from ASTM F2394-07 (described in next section). Drug adherence and loss on the way to the vessel was tested in vitro by Kelsch et al.8 Drug loss upon passage through a blood-filled hemostatic valve and guiding catheter for one minute in stirred blood at 37 °C was investigated. Urea-based DCB lost 26 ± 3% and iopromide-based DCB lost 36 ± 11% of the total amount on the balloon.8
In conclusion for the simulated use of DCB, the total drug delivery upon dilation is different for the used hydrogels simulating the vessel wall. Calcium alginate hydrogel as the vessel model showed the highest PTX delivery upon dilation. The wash-off from the alginate hydrogel was high (drug release after 1 min by a simulated blood stream: 41.2 ± 14.2%). However, 21.4 ± 10.7% of the drug diffused into the hydrogel compartment. The silicone tube showed the least amount of wash-off (<1%) from the vessel model after 1 min, but it is quite different to natural vessels. Poly(VEImBr) hydrogels as vessel models were difficult to analyze. In the case of PAAm as the vessel model, only 20% of PTX could be delivered upon dilation.
3.2. Simulated use of DCB in the vessel-simulating flow-through cell after passage through an in vitro vessel model according to ASTM F2394-07
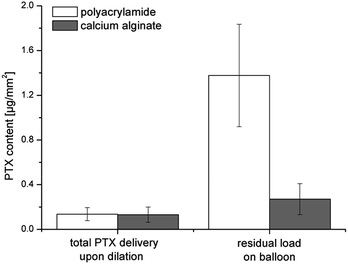
In order to simulate the implantation process, the vessel-simulating flow-through cell was combined with a model coronary artery pathway to estimate drug loss and transfer as well as particle release. Cetpyrsal-based DCBs were manually advanced through a tortuous vessel path, consisting of a guiding catheter with a guide wire. Calcium alginate and polyacrylamide hydrogels were used as tissue models for the simulated use in an in vitro model (Fig. 4). The obtained results can be compared with the data from Petersen et al.11 In their study, they also used the anatomic model according to ASTM F2394-07 with a silicone tube as the vessel model.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, w/w) coated in a folded condition. With the balloon coated in an expanded condition, the PTX transfer in the silicone tube was lower (5–15%).11 Here, the used balloon catheters were coated in an expanded condition. Seidlitz et al. used pure PTX-coated balloons and showed PTX transfer rates to gel below 1% (calcium alginate as vessel model).12 In their study, they also used a model of a coronary artery pathway to investigate drug loss and drug transfer to the gel. However, in our study with the novel DCB coating more PTX was delivered upon dilation (calcium alginate: 6.4 ± 3.8% compared to below 1%). In conclusion, the PTX transfer upon dilation depends on the coating of the balloon and the used vessel model simulating the vessel wall.
50, w/w) coated in a folded condition. With the balloon coated in an expanded condition, the PTX transfer in the silicone tube was lower (5–15%).11 Here, the used balloon catheters were coated in an expanded condition. Seidlitz et al. used pure PTX-coated balloons and showed PTX transfer rates to gel below 1% (calcium alginate as vessel model).12 In their study, they also used a model of a coronary artery pathway to investigate drug loss and drug transfer to the gel. However, in our study with the novel DCB coating more PTX was delivered upon dilation (calcium alginate: 6.4 ± 3.8% compared to below 1%). In conclusion, the PTX transfer upon dilation depends on the coating of the balloon and the used vessel model simulating the vessel wall.
| PAAm > 10 μm | PAAm > 25 μm | Calcium alginate > 10 μm | Calcium alginate > 25 μm | |
|---|---|---|---|---|
| After track | 230 ± 126 | 33 ± 16 | 580 ± 308 | 56 ± 27 |
| After expansion | 4 ± 1 | 1 ± 1 | 9 ± 1 | 1 ± 1 |
| Sum | 234 ± 127 | 34 ± 17 | 589 ± 309 | 57 ± 28 |
Using calcium alginate as the vessel model, a total of 589 ± 309 particles (>10 μm) per mm2 were analyzed. Contained particles >25 μm per mm2 were detected in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (57 ± 28). In the second test series using PAAm, the expected sum of particles was decreased (234 ± 127 (>10 μm) per mm2, 34 ± 7 (>25 μm) per mm2 balloon surface). Petersen et al. described that DCB based on Cetpyrsal generated a lower quantity of particles (expanded condition: 280 ± 91 particles (>10 μm) per mm2 balloon surface) compared to commercially available DCB using a urea-based coating (329 ± 161 particles (>10 μm) per mm2 balloon surface).11 Amounts of particles generated from the PTCA balloon catheters by comparing two modified lubricous polymeric hydrogel coatings used at various thicknesses were demonstrated by Babcock et al.28 In their study, a submicron coating (dry thickness of 0.5 μm) generates far fewer particulates than the micron coating (dry thickness of 2 μm) on the same substrate in a standard anatomic model adapted from ASTM F2394-07.28
10 (57 ± 28). In the second test series using PAAm, the expected sum of particles was decreased (234 ± 127 (>10 μm) per mm2, 34 ± 7 (>25 μm) per mm2 balloon surface). Petersen et al. described that DCB based on Cetpyrsal generated a lower quantity of particles (expanded condition: 280 ± 91 particles (>10 μm) per mm2 balloon surface) compared to commercially available DCB using a urea-based coating (329 ± 161 particles (>10 μm) per mm2 balloon surface).11 Amounts of particles generated from the PTCA balloon catheters by comparing two modified lubricous polymeric hydrogel coatings used at various thicknesses were demonstrated by Babcock et al.28 In their study, a submicron coating (dry thickness of 0.5 μm) generates far fewer particulates than the micron coating (dry thickness of 2 μm) on the same substrate in a standard anatomic model adapted from ASTM F2394-07.28
4. Conclusions
Drug-coated balloon catheters are an alternative for coronary and peripheral artery disease. Based on the limited number of published results of in vitro characterization of drug coated balloons, there is a need for further research. Novel PTX-coated balloons using ionic liquid Cetpyrsal as an additive for the in vitro study were applied. Drug delivery upon dilation in different tissue models (calcium alginate, poly(VEImBr) and PAAm) using a vessel-simulating flow-through cell was investigated and compared to a silicone tube as the tissue model. The highest PTX delivery upon dilation was achieved with calcium alginate as the vessel model (about 60%). However, a total PTX delivery upon dilation of 20% was determined with polyacrylamide as vessel model. The used vessel models showed seemingly various adhesion properties, thus the PTX wash-off quantities during simulated blood flow were different. The silicone tube showed the lowest amount of wash-off (<1%) from the vessel model after 1 min simulated blood stream. The highest drug wash-off (release) was achieved with calcium alginate as vessel model. Moreover, the diffusion of PTX into the vessel wall occurs at various rates, which may be related to the cross-linker content of the hydrogels. In addition to solubility and thus diffusion of PTX, the hydrogel material as well as the coating was crucial for drug transfer from the balloon into the vessel wall when compared to reported data. Furthermore, the vessel-simulating flow-through cell was combined with a model coronary artery pathway to simulate an anatomic implantation process. Vast amounts of the coated drug were lost during a simulated artery pathway. Only a small fraction of the total loads of PTX were delivered upon dilation. Similar transfer rates for PTX upon dilation were achieved with calcium alginate and PAAm as vessel models. The crucial drug delivery upon dilation was examined with the aid of different hydrogel materials to evaluate the in vitro research. These are important data for the in vivo application.Acknowledgements
The authors thank Jana Unger for skillful assistance in synthesizing the ionic liquids and Dr Thomas Reske as well as Philip Wahl for their expert technical assistance. We also acknowledge Biotronik SE & Co KG for a generous supply of the uncoated balloon catheters. For fruitful discussions within the REMEDIS network we thank the group of Prof. Dr Katrin Sternberg, IBMT University of Rostock. Furthermore, we would like to thank Dr Anne Seidlitz, Dr Beatrice Semmling and Prof. Dr Werner Weitschies for cooperation and design of the vessel-simulating flow-through cell. Financial support by Bundesministerium für Bildung und Forschung (BMBF) within REMEDIS “Höhere Lebensqualität durch neuartige Mikroimplantate” (FKZ:03IS2081) is gratefully acknowledged.References
- A. Lupi, A. Rognoni, G. G. Secco, I. Porto, F. Nardi, M. Lazzero, L. Rossi, R. Parisi, R. Fattori, G. Genoni, R. Rosso, P. R. Stella, I. Sheiban, L. Bolognese, F. Liistro, A. S. Bongo and P. Agostoni, Int. J. Cardiol., 2013, 168, 4608–4616 CrossRef PubMed.
- M. Krokidis, S. Spiliopoulos, K. Katsanos and T. Sabharwal, Cardiovasc. Intervent. Radiol., 2013, 36, 281–291 CrossRef PubMed.
- R. Waksman and R. Pakala, Circ.: Cardiovasc. Interventions, 2009, 2, 352–358 CrossRef CAS PubMed.
- I. Narbute, S. Jegere, I. Kumsars, I. Mintale, I. Zakke, K. Bumeistere, D. Sondore, A. Grave and A. Erglis, Medicina, 2011, 47, 536–543 Search PubMed.
- A. Posa, N. Nyolczas, R. Hemetsberger, N. Pavo, O. Petnehazy, Z. Petrasi, G. Sangiorgi and M. Gyongyosi, Cathet. Cardiovasc. Interv., 2010, 76, 395–403 CrossRef PubMed.
- J. P. Loh and R. Waksman, JACC: Cardiovascular Interventions, 2012, 5, 1001–1012 CrossRef PubMed.
- F. X. Kleber, H. Rittger, K. Bonaventura, U. Zeymer, J. Wohrle, R. Jeger, B. Levenson, S. Mobius-Winkler, L. Bruch, D. Fischer, C. Hengstenberg, T. Porner, D. Mathey and B. Scheller, Clin. Res. Cardiol., 2013, 102, 785–797 CrossRef CAS PubMed.
- B. Kelsch, B. Scheller, M. Biedermann, Y. P. Clever, S. Schaffner, D. Mahnkopf, U. Speck and B. Cremers, Invest. Radiol., 2011, 46, 255–263 CrossRef CAS PubMed.
- B. Scheller, U. Speck, C. Abramjuk, U. Bernhardt, M. Bohm and G. Nickenig, Circulation, 2004, 110, 810–814 CrossRef CAS PubMed.
- S. Petersen, I. Minrath, S. Kaule, J. Kocher, K. P. Schmitz and K. Sternberg, Coatings, 2013, 3, 253–267 CrossRef CAS PubMed.
- S. Petersen, S. Kaule, F. Stein, I. Minrath, K. P. Schmitz, U. Kragl and K. Sternberg, Mater. Sci. Eng., C, 2013, 33, 4244–4250 CrossRef CAS PubMed.
- A. Seidlitz, N. Kotzan, S. Nagel, T. Reske, N. Grabow, C. Harder, S. Petersen, K. Sternberg and W. Weitschies, PLoS One, 2013, 8, e83992 Search PubMed.
- J. Bandomir, A. Schulz, S. Taguchi, L. Schmitt, H. Ohno, K. Sternberg, K.-P. Schmitz and U. Kragl, Macromol. Chem. Phys., 2014, 215, 716–724 CrossRef CAS PubMed.
- K. Bica, C. Rijksen, M. Nieuwenhuyzen and R. D. Rogers, Phys. Chem. Chem. Phys., 2010, 12, 2011–2017 RSC.
- A. Seidlitz, S. Nagel, B. Semmling, N. Grabow, H. Martin, V. Senz, C. Harder, K. Sternberg, K.-P. Schmitz, H. K. Kroemer and W. Weitschies, Eur. J. Pharm. Biopharm., 2011, 78, 36–48 CrossRef CAS PubMed.
- C. Dimario, N. Meneveau, R. Gil, P. Dejaegere, P. J. Defeyter, C. J. Slager, J. Roelandt and P. W. Serruys, Am. J. Cardiol., 1993, 71, D54–D61 CrossRef.
- W. Schmidt and P. Lanzer, in Catheter-Based Cardiovascular Interventions, ed. P. Lanzer, Springer-Verlag Berlin, Heidelberg, P. Lanzer edn, 2013, pp. 445–472 Search PubMed.
- R. T. Liggins, W. L. Hunter and H. M. Burt, J. Pharm. Sci., 1997, 86, 1458–1463 CrossRef CAS PubMed.
- T. Konno, J. Watanabe and K. Ishihara, J. Biomed. Mater. Res., Part A, 2003, 65A, 209–214 CrossRef CAS PubMed.
- Y. L. Khmelnitsky, C. Budde, J. M. Arnold, A. Usyatinsky, D. S. Clark and J. S. Dordick, J. Am. Chem. Soc., 1997, 119, 11554–11555 CrossRef CAS.
- N. A. Peppas, B. V. Slaughter, M. A. Kanzelberger, K. Matyjaszewski and M. Möller, in Polymer Science: A Comprehensive Reference, Elsevier, Amsterdam, 2012, vol. 9, pp. 385–395 Search PubMed.
- Y. G. Kim, C. H. Lee and Y. C. Bae, Fluid Phase Equilib., 2014, 361, 200–207 CrossRef CAS PubMed.
- J. K. Kim, H. J. Kim, J. Y. Chung, J. H. Lee, S. B. Young and Y. H. Kim, Arch. Pharmacal Res., 2014, 37, 60–68 CrossRef CAS PubMed.
- A. Ruebben, J. Boeing and N. Weiss, J. Intervent. Cardiol., 2010, 5, 74–76 CrossRef.
- M. Afari and J. Granada, Endovascular Today, 2012, 53–58 Search PubMed.
- T. Heilmann, C. Richter, H. Noack, S. Post, D. Mahnkopf, A. Mittag, H. Thiele and H.-R. Figulla, European Cardiology Review, 2010, 6, 40–44 CrossRef.
- B. Cortese and A. Bertoletti, Int. J. Cardiol., 2012, 161, 4–12 CrossRef PubMed.
- D. E. Babcock, R. W. Hergenrother, D. A. Craig, F. D. Kolodgie and R. Virmani, Biomaterials, 2013, 34, 3196–3205 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |