Open Access Article
Open Access ArticlePhotoswitchable stable charge-distributed states in a new cobalt complex exhibiting photo-induced valence tautomerism†
Michael
Slota
*ab,
Marian
Blankenhorn
a,
Eric
Heintze
a,
Minh
Vu
a,
Ralph
Hübner
a and
Lapo
Bogani
*b
a1. Physikalisches Institut, University of Stuttgart, Pfaffenwaldring 57, 70569 Stuttgart, Germany. E-mail: michael.slota@pi1.physik.uni-stuttgart.de; Tel: +49 711 68564891
bDepartment of Materials, University of Oxford, 16 Parks Road, OX1 3PH, Oxford, UK. E-mail: lapo.bogani@materials.ox.ac.uk; Tel: +44 (0)1865 283341
First published on 3rd June 2015
Abstract
We report the synthesis and magnetic and photomagnetic behaviour of a novel valence tautomeric cobalt complex, [Co(3,5-dbbq)2(μ-bpym)] (1) (3,5-dbbq = 3,5-di-tert-butyl-1,2-benzoquinone and μ-bpym = 2,2′-bipyrimidine). The synthesis is performed by reacting Co2(CO)8 and μ-bpym in the presence of the ligand 3,5-dbbq in a mixed solvent under inert atmosphere. The magnetic behavior clearly shows the presence of electron transfer from the catecholate ligand to the cobalt center, producing valence tautomers of [CoII(SQ)2] with a transition temperature (T1/2) of 215 K. Photomagnetic studies, performed via both SQUID magnetometry and X-band electron paramagnetic resonance, show the clear presence of photoinduced valence tautomerism, at temperatures considerably higher than previous systems. A metastable charge distribution is observed, strengthening previous investigations on the character of mixed valence ligands. Entropy-driven valence tautomeric interconversion is observed, and drives the transition to the most stable charge distribution. The complex has the ability to coordinate and can be used as a photoswitchable building block, with the photomagnetic characterisation evidencing a metastable state lifetime of the photo-induced valence tautomeric process of ca. 2.9 × 104 s below 20 K. The observed yields are higher than ones in similar systems, showing that tiny changes in the molecular structures may have a huge impact.
1 Introduction
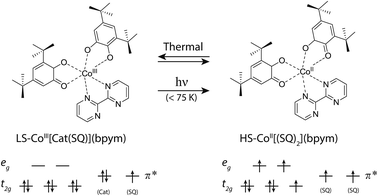
The effect of magnetism on light has been fascinating scientists for more than 170 years, since the Faraday effect was discovered,1 and since 1900 also the actions of light on the magnetic properties has found more and more attention.2 Modern chemistry and new physical investigation methods have allowed clear evidence of phenomena where the magnetization state can be tuned back and forth by external stimuli, such as light, as well as temperature, pressure, current or chemical doping.3–14 For the development of new nanodevices, the individual building blocks need to be reduced down to the molecular level and need to be addressed with high precision. This thus necessitates systems with molecular bistability that can be switched using light. Recent attempts at creating multifunctional and bistable molecular complexes that possess a switchable magnetic functionality have led to novel methods that switch the magnetic properties of nanomagnetic systems, such as single-molecule magnets15,16 and single chain magnets.17,18 In all these effects some prominent examples of phenomena that trigger the molecular magnetic bistability are complexes which display valence tautomerism (VT),15,19–22 spin crossover (SCO) behavior23–25 or photoisomerization processes.26 VT is a particularly interesting phenomenon, discovered thirty-five years ago by Buchanan and Pierpont,27 that is constituted by an intramolecular electron transfer (ET) process. These compounds usually undergo intramolecular ET between the coordinated metal center and the surrounding ligands, while this phenomenon is often accompanied by SCO behaviour of the metal ion, owing to the closely spaced d levels. The coordination compounds of the Co–catecholate/semiquinone families are known to display VT, and several examples have been reported, attracting considerable interest.7,10,21,22,28–32 Usually, the VT mechanism implies electron transfer from the ligands, leading to the interconversion between the low-spin CoIII–catechole (Cat) and high-spin CoII–semiquinone (SQ) triggered by the presence of redox-active quinone ligands. In these systems, the valence tautomeric process can be schematised as follows:| [CoIII(μ-bpym)(Cat)(SQ)]LS ⇌ [CoII(μ-bpym)(SQ)2]HS | (1) |
It has been shown that this process can be initiated by light-induced electron transfer,19,33–36 which offers an interesting experimental ground for the investigation of photo-induced effects on spin centers. The structural properties are connected to the magnetic bistability providing a fertile ground for the study of molecular and crystal engineering. It is reported that this molecular transition is accompanied with a contraction of the coordination-sphere.21 The use of nitrogen-substituted ligands with multi-chelating properties is particularly appealing, because the presence of two binding sites can be used to bind to other metals, and could, in perspective, allow obtaining various phototunable polymetallic compounds, where the Co VT complex is used as a building block in a rational process.
Here we describe the synthesis and photomagnetic and EPR properties of a novel photoswitchable VT complex. We present a compound of the Co-catecholate/semiquinone family that can be used as a phototunable building block for constructing multifunctional valence tautomeric systems. The present system differs from other examples of VT complexes, and in particular from the previously-reported parent system21 containing 3,6-di-tert-butyl-1,2-benzoquinone and 2,2′-bipyrimidine by the presence of an exchanged 3,5-di-tert-butyl-1,2-benzoquinone ligand. Hereafter we show that the new system displays enhanced properties, and provide a complete investigation including electron paramagnetic resonance and magnetic measurements under irradiation at different wavelengths and temperatures. By the combined use of these techniques, a metastable state lifetime of the photo-induced valence tautomeric process of ca. 2.9 × 104 s below 20 K is found, and we determine photoswitching yields much higher than ever presented before, reaching 98%. The results point at the drastic changes that even small variations in the substitution positions in the ligands may induce in such molecular systems.
2 Materials and methods
2.1 Synthesis
The VT complex [Co(μ-bpym)(3,5-dbbq)2] (1) was obtained following procedures similar to those reported in the literature.37 All chemicals (Co2(CO)8, 2,2′-bipyrimidine (= bpym) and 3,5-di-tbutyl-1,2-benzoquinone (= 3,5-dbbq), were purchased from Sigma-Aldrich and used without further purification except the solvents. The solvents (toluene and hexane) were purified with the standard procedures and degassed via the repeated freeze–pump method38 and stored under argon. The synthesis and recrystallization of [Co(μ-bpym)(3,5-dbbq)2] was performed under inert gas (Ar) using the standard reversed flow Schlenk technique. Elemental analyses were carried out with a PerkinElmer Analyzer 240.For the synthesis, Co2(CO)8 (85.5 mg, 0.25 mmol) and 2,2′-bipyrimidine (87 mg, 0.55 mmol) were stirred for 10 min in 20 ml toluene. Then 3,5-dbbq (220 mg, 2 mmol) in 15 ml toluene was added. The blue mixture was then stirred for an additional 3 h, and then concentrated under reduced pressure and stored overnight at 4 °C. The blue precipitate was then filtered and washed with hexane. The complex was recrystallized from a mixture of toluene (10 ml) and hexane (30 ml). Filtration of the mixture produces a dark-blue solid in 50% yield. Found: C, 66.20%; H, 6.85%; N, 8.23%. Calculated for C36H46CoN4O4: C, 65.74%; H, 7.05%; N, 8.52%.
2.2 Magnetic and photomagnetic measurements
All magnetic measurements were performed using a commercially available SQUID magnetometer, MPMS-XL7 by QuantumDesign. We prepared the sample in two different ways. First, 2.9 mg of 1 in powder form was filled into the cap of an agar agar capsule (5 mm diameter) of 37 mg mass. Second, a 7.0 mg sample was pressed into a thin pellet of 5 mm diameter, on which laser power dependent measurements were conducted. To avoid cracking, the pellet was placed between two Suprasil quartz glass platelets of 1 mm thickness and 5 mm diameter each. Both samples were fixed in a plastic straw. In order to obtain thinner samples, we tested dissolving the molecule in dichloroethane and then letting the solution dry on top of a glass plate. However, during this process, the sample lost its valence tautomeric property and, accordingly, its photo- and thermally-switchable phase transition (Fig. SI1†), underlying the fact that valence tautomeric complexes are quite sensitive to their environment.36,39,40To illuminate the sample and measure its photomagnetic properties on the SQUID magnetometer we developed a home-made system that allows excellent collimation of light onto the sample via an optical fiber. Light from a 532 nm diode laser (15 mW maximum output power) is guided via a single-mode fiber through a hollow rod into the sample chamber. The output power was controlled via a set of neutral density filters. Prior to each measurement the maximum output power behind the optical fiber end was set to distinct values between 0.2 mW and 5.4 mW. Owing to the usually large extinction coefficient, only very thin samples were investigated. Later in the paper we will discuss the crucial role of sample preparation for the investigation of light-induced properties.
All magnetic data were obtained at a field of 5000 Oe within 2 K and 300 K. The sweep rates were set to 0.5 K min−1 below 100 K and 2.5 K min−1 above. All data were corrected for the diamagnetic background of the sample and sample holders, as independently determined.
2.3 EPR and photospectroscopic measurements
Temperature-dependent X-Band (9.5 GHz) electron paramagnetic resonance (EPR) spectroscopy studies were performed on a Bruker EMXplus system using a universal X-Band resonator ER 4102ST between 300 K and 3 K in an Oxford ESR910 cryostat. Approximately 0.2 mg of a powder sample was placed in an open polycarbonate capsule (4 mm diameter) mounted at the bottom of an open quartz glass tube, which ensures good thermal contact. To guide light onto the sample, we fixed an optical fiber with 1 mm core diameter about 4 mm above the sample. The calibrated output power of the utilized LOT-QuantumDesign 150 W Xe arc lamp with a 532 nm bandpass filter (10 nm bandwidth) was around 1.5 mW behind the optical fiber. Via a set of bandpass filters (40 nm bandwidth each), wavelength dependent absorption between 400 nm and 850 nm was investigated.3 Results and discussion
3.1 Magnetic properties and ESR spectroscopy without irradiation
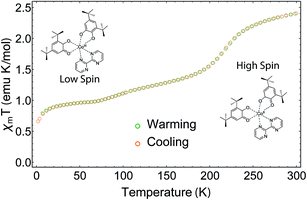
The magnetic moment of a pellet sample of 1 was recorded during cooling and warming between 2 K and 300 K at a field of 5000 Oe (Fig. 2). The pellet form ensures that no rearrangement of the sample takes place during the measurement. At 300 K, the χmT value, where χm corresponds to the molar magnetic susceptibility and T is the temperature, is 2.41 emu K mol−1 and does not reach a saturation point. A χmT around 2.5 emu K mol−1 is typical for HS-CoII complexes,28 which indicates a HS ground state at room temperature. When decreasing the temperature, the sample displays a rapid decrease in χmT centered at T1/2 = 215 K. This is due to the phase transition from HS-CoII to LS-CoIII, where a charge is transferred from the HS-CoII ion to the SQ ligand, which is usually accompanied by a contraction of the coordination sphere.21 The rapid decrease is followed by a gradual decrease below 200 K until the system reaches a shoulder-shaped plateau of 0.97 emu K mol−1 at 70 K. In contrast to similar Co-based VT compounds, the plateau value does not correspond to the typical spin-1/2 value of 0.38 emu K mol−1 for g = 2. This would be expected for a total HS to LS transition, where the paramagnetic signal originates solely from the remaining SQ radicals. The intriguing result could be caused by the presence of a residual amount of a HS-CoII impurity, which has already been discussed for some Co VT compounds, but at a much lesser extent.28 Any other paramagnetic impurities can safely be neglected, as the elemental analysis is coincident with calculations. Moreover, the increased χmT value could also be due to the chosen slow temperature sweep rates, where rearrangements of the charge distributions may occur.28 Furthermore, we assume that ferromagnetic exchange mechanisms between the SQ π*-electrons and the residual HS-CoII ions could also play a role here.29 For temperatures below 10 K, the value drops well below 0.9 emu K mol−1, which we attribute to antiferromagnetic exchange interactions between the spins of the SQ ligands.28When warming up from 2 K to 300 K, the sample regains its initial susceptibilities. Even after many thermal cycles, the sample does not show any deterioration of the magnetic signal, proving that the switching is fully thermally-reversible.
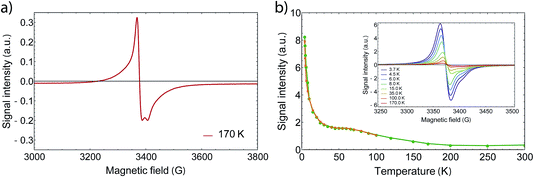
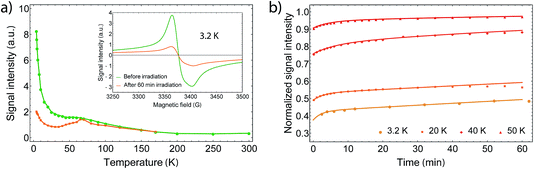
In order to gain further information on the spin state of the molecule, we conducted EPR spectroscopy in the X-band regime for temperatures between 3 K and 300 K (Fig. 3), where no differences between cooling and warming are observed. At 260 K, we observe a small derivative signal centered at 3385(3) G with g = 2.006 (see Fig. SI2†). By cooling down below 220 K, a second derivative signal overlays the spectrum, which is centered at 3376(1) G with g = 2.001 (see Fig. SI3†), and becomes directly visible below 170 K. Because SQUID measurements give T1/2 = 215 K for the HS to LS transition, we assign the transition at 3376 G to the organic radical SQ in the formed LS-CoIII(Cat)(SQ) species. Owing to fast relaxation times, CoII(SQ)2 complexes are reported to be EPR-silent34 and thus not observable at higher temperatures. As both signals show a similar temperature dependence when corrected for the Boltzmann population effect, the second signal may arise from unavoidable impurities or anisotropies of the microcrystalline samples. Therefore it is valid to analyse the EPR data by collecting the signal maxima and minima. Upon further lowering the temperature, the 3376 G signal dominates the spectrum, which therefore gives the proof of an increasing amount of spin-1/2 species. Further we could also observe a small plateau around 70 K, which falls in line with the SQUID plateau. Below 70 K, when the thermal phase-transition is complete, the signal rises exponentially with temperature due to Boltzmann distribution of states.
At low temperatures, we observed a small derivative signal centered at 1700 G, which is approximately 500 times smaller than the sharp spin-1/2 signal (Fig. SI4†). We attribute this second signal to a ΔM = ±2 transition with g = 4, which arises from the exchange coupling of the radical ligands.
3.2 The photomagnetic processes
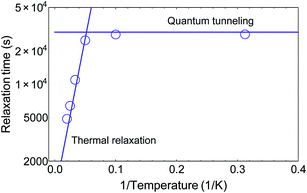
It has been shown that, after having been cooled down, VT compounds can be switched back into the HS state via irradiation with visible light. Below a certain blocking temperature, the photoexcited metastable state is maintained for very long times, which range from minutes to several days.22 Thermal relaxation drives the system back into a ground state, which can be described by an Arrhenius lawτ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Δ/kBT) exp(Δ/kBT) | (2) |
Comparable measurements with same laser power on a pressed pellet sample resulted in an approximately four times lower yield. As mentioned before, this could be due to the partially quenched VT induced by pressure or the lower surface to volume ratio compared to powder. Still, pellets offer the possibility to quantitatively investigate the effect of laser power, owing to the defined geometry. Due to the Beer–Lambert law and a Gaussian beam profile the excitation fraction scales non-linearly with the laser power (Fig. SI5†). Our investigation shows that increasing the laser power beyond 2 mW results in increasingly smaller changes of the excitation efficiency. Thus, significantly higher yields can only be achieved via sample preparation in powder form or changing to wavelengths at which the sample shows a lower extinction coefficient (see discussion below).
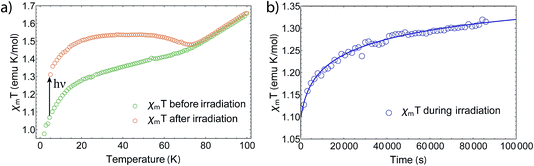
Furthermore, we also noticed a kink appearing around 12 K (Fig. 4a) after exciting the sample at 5 K and increasing T. This kink is also reported for the 3,6-tert-butyl derivate,21 but has not been discussed yet and its origin is still unknown. After heating the sample to room temperature and cooling down to 5 K again, the complex regains its initial magnetic moment, showing full thermal reversibility with no measurable degeneration. However, we noticed that sample aging (several days) in an external field results in up to 10% changes of the magnetic moment, which we assume to be caused by different trapping patterns of the differently charged ligands.28 By removing the field at room temperature, the magnetization relaxes slowly towards the starting value. The photomagnetic properties are maintained nevertheless.
In order to determine the relaxation behavior, we measured the time evolution of the magnetic moment under irradiation with 532 nm light at 5 K (Fig. 4b). Due to photoconversion, the time evolution displays an increasing and saturating behavior, whereby the photostationary limit has not been reached even after 24 h of irradiation. We fitted the relaxation times via a first-order stretched exponential law given by
a(t) = a0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−(t/τ)β), exp(−(t/τ)β), | (3) |
By fitting we determined the lifetime τ to be 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 ± 5000 s at 5 K. Thereby, we estimated the heating during irradiation to be ΔT = 2.45 K, so that the final χmT value matches with the one after irradiation at 5 K. However, it should be noted that the fit does not describe the data well due to thermal instabilities during the measurement and the nonlinear response of χm at low Ts.
700 ± 5000 s at 5 K. Thereby, we estimated the heating during irradiation to be ΔT = 2.45 K, so that the final χmT value matches with the one after irradiation at 5 K. However, it should be noted that the fit does not describe the data well due to thermal instabilities during the measurement and the nonlinear response of χm at low Ts.
The magnetic measurements thus prove that the compound undergoes a long-lived transition into a metastable state.
In order to understand the relaxation mechanisms, we measured the derivative signal intensity at different temperatures for 60 min after 60 min of irradiation (Fig. 5b). The relaxation behavior was fitted using two single-exponential laws
| A(t) = 1 − A1(0)exp(−t/τ1) − A2(0)exp(−t/τ2), | (4) |
3.3 Investigation of possible back-switching
Eventually, we examined whether it is possible to revert the light-induced switching from LS to HS via illumination of near infrared light at 850 nm. Such behavior has been reported for similar Co VT compounds.42,47 While 532 nm light drives a LMCT process, 850 nm could induce a HS–LS transition via MLCT. To see a possible back-switching effect, we first irradiated our sample at 3.7 K in the X-Band spectrometer at 532 nm for 70 min and directly exchanged the bandpass filter with a 850 nm one. If back-switching is present, the X-Band EPR signal would increase due to an increasing amount of LS-CoIII(Cat)(SQ) species. However, the strong opposite is the case and the signal decreased drastically (see Fig. 7), where after 30 min of irradiation at 850 nm we even reached a total excitation yield of 98%, and this without the use of a pulsed laser source. Therefore, we can exclude a back-switching behavior in this wavelength region for our sample. The higher conversion efficiency can be related to the lower absorption coefficient in the near infrared as determined from UV/Vis/NIR spectroscopy (see Fig. SI7†) and accordingly with a resulting higher penetration depth, so that a higher amount of photons may reach underlying molecules. Also, the output power behind the fiber was strongly enhanced by the four times higher bandwidth, which contributed to the higher yield.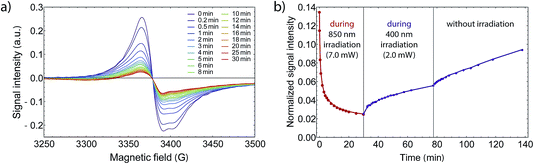 | ||
| Fig. 7 (a) EPR signal intensity while irradiating with 850 nm light and 7 mW light power, after first irradiating for 70 min at 532 nm and 1.5 mW. (b) Wavelength dependent performance of the photoabsorption process at 3.75 K. After the first irradiation at 532 nm, the normalized signal was reduced from 1.0 to 0.14, whereby the signal change is comparable to SI6.† In contrast to some other Co VT compounds that show back switching behavior, the excitation is enhanced. The signal decreases even further down close to 0.02, which corresponds to a net excitation of 98%. By changing the wavelength to 400 nm at 1.5 mW, the signal increases again. As the signal increases faster after turning off the light source, the increase during irradiation at 400 nm is due to the fact that the relaxation goes faster than the excitation at this wavelength. | ||
When studying back-switching processes, a larger output power at the de-excitation wavelength is crucial. Otherwise, because the absorption coefficient changes with wavelength, the net excitation fraction may decrease due to counteracting thermal and quantum tunneling relaxation processes. This was exactly the case when we irradiated the sample with 400 nm subsequently at a lower output power. Moreover, from the UV/Vis/NIR spectrum the absorption at 400 nm is larger compared to 850 nm and accordingly the penetration depth is smaller. All in all, we observed an increase of the EPR signal after 45 min of irradiating at 400 nm, which would seem to be a light-induced de-excitation. However, since the signal decreased slightly faster without irradiation, we explain the decrease at 400 nm to be completely caused by molecular relaxation processes. Finally we did not detect any back-switching from HS to LS between 400 nm and 850 nm.
4 Conclusion
In conclusion we presented the synthesis and magnetic and photomagnetic properties of a novel Co-based VT compound with improved properties. The photomagnetic transition and relaxation process of the complex 1 was thoroughly investigated by SQUID magnetometry and EPR spectroscopy at low temperatures. We were able to identify two relaxation regimes, a temperature independent one due to quantum tunneling below 20 K and a thermal relaxation that follows an Arrhenius law. Furthermore, we could achieve an almost full excitation at 850 nm without the use of a pulsed laser. We showed the relevance of sample preparation in order to achieve the best conversion efficiency and demonstrated extremely appealing properties of the compound in terms of temperature and efficiency. We also investigated the power dependence of the effects, showing that laser powers above 2 mW produce only marginal efficiency increases on the SQUID magnetometer. These results show that the novel complex 1 is a superior building block, showing a significantly higher critical temperature TLIESST. Its use in the rational design of novel molecular magnets opens the path to molecular photoswitchable systems and multifunctional materials. Finally, the large efficiency observed now allows pulsed transient EPR measurements to be performed under light irradiation, possibly leading to a clearer understanding of the fundamental processes in VT systems.Acknowledgements
We thank the Royal Society via the University Research Fellowship and the University Research Grant, the AvH Stiftung (Sofja Kovalevskaja award) and the European Research Council via the grant ERC-StG-338258-”OptoQMol”.References
- M. Faraday, Philos. Trans. R. Soc. London, 1846, 136, 1–20 CrossRef.
- J. H. Hart, Am. J. Sci., 1900, 10, 66–73 CrossRef.
- C. Cervetti, E. Heintze and L. Bogani, Dalton Trans., 2014, 4220–4232 RSC.
- O. Sato, J. Tao and Y.-Z. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2152–2187 CrossRef CAS PubMed.
- A. Caneschi, A. Dei, F. Fabrizi De Biani, P. Gütlich, V. Ksenofontov, G. Levchenko, A. Hoefer and F. Renz, Chem.–Eur. J., 2001, 7, 3926–3930 CrossRef CAS.
- F. Matsukura, Y. Tokura and H. Ohno, Nat. Nanotechnol., 2015, 10, 209–220 CrossRef CAS PubMed.
- H. Das, F. Weisser, D. Schweinfurth, C.-Y. Su, L. Bogani, J. Fiedler and B. Sarkar, Chem.–Eur. J., 2010, 16, 2977–2981 CrossRef CAS PubMed.
- D. Schweinfurth, F. Weisser, D. Bubrin, L. Bogani and B. Sarkar, Inorg. Chem., 2011, 50, 6114–6121 CrossRef CAS PubMed.
- A. Droghetti and S. Sanvito, Phys. Rev. Lett., 2011, 107, 047201 CrossRef CAS PubMed.
- P. L. Gentili, L. Bussotti, R. Righini, A. Beni, L. Bogani and A. Dei, Chem. Phys., 2005, 314, 9–17 CrossRef CAS.
- R. Moroni, R. Buzio, A. Chincarini, U. Valbusa, F. B. de Mongeot, L. Bogani, A. Caneschi, R. Sessoli, L. Cavigli and M. Gurioli, J. Mater. Chem., 2008, 18, 109–115 RSC.
- C. de Julián Fernández, G. Mattei, E. Paz, R. L. Novak, L. Cavigli, L. Bogani, F. J. Palomares, P. Mazzoldi and A. Caneschi, Nanotechnology, 2010, 21, 165701 CrossRef PubMed.
- L. Bogani, L. Cavigli, C. de Julin Fernndez, P. Mazzoldi, G. Mattei, M. Gurioli, M. Dressel and D. Gatteschi, Adv. Mater., 2010, 22, 4054–4058 CrossRef CAS.
- L. Cavigli, C. de Julin Fernndez, D. Gatteschi, M. Gurioli, C. Sangregorio, G. Mattei, P. Mazzoldi and L. Bogani, J. Magn. Magn. Mater., 2007, 316, e798–e801 CrossRef CAS.
- O. Sato, Proc. Jpn. Acad., Ser. B, 2012, 88, 213–225 CrossRef CAS PubMed.
- C. Mathonière, H. J. Lin, D. Siretanu, R. Clérac and J. M. Smith, J. Am. Chem. Soc., 2013, 135, 19083–19086 CrossRef PubMed.
- E. Heintze, F. El Hallak, C. Clauß, A. Rettori, M. G. Pini, F. Totti, M. Dressel and L. Bogani, Nat. Mater., 2013, 12, 202–206 CrossRef CAS PubMed.
- T. Liu, H. Zheng, S. Kang, Y. Shiota, S. Hayami, M. Mito, O. Sato, K. Yoshizawa, S. Kanegawa and C. Duan, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- O. Sato, A. Cui, R. Matsuda, J. Tao and S. Hayami, Acc. Chem. Res., 2007, 40, 361–369 CrossRef CAS PubMed.
- D. N. Hendrickson and P. G. Gortlandt, Top. Curr. Chem., 2004, 234, 63–95 CrossRef CAS.
- J. Dai, S. Kanegawa, Z. Li and S. Kang, Eur. J. Inorg. Chem., 2013, 4150–4153 CrossRef CAS.
- R. D. Schmidt, D. A. Shultz, J. D. Martin and P. D. Boyle, J. Am. Chem. Soc., 2010, 132, 6261–6273 CrossRef CAS PubMed.
- J.-F. Létard, P. Guionneau and L. Goux-Capes, Top. Curr. Chem., 2004, 235, 221–249 CrossRef.
- R. Bertoni, M. Cammarata, M. Lorenc, S. F. Matar, J.-F. Létard, H. T. Lemke and E. Collet, Acc. Chem. Res., 2015, 48, 774–781 CrossRef CAS PubMed.
- A. Hauser, Top. Curr. Chem., 2004, 234, 155–198 CrossRef CAS.
- S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sönnichsen, F. Tuczek and R. Herges, Science, 2011, 331, 445–448 CrossRef CAS PubMed.
- R. M. Buchanan and C. G. Pierpont, J. Am. Chem. Soc., 1980, 102, 4951–4957 CrossRef CAS.
- M. Affronte, A. Beni, A. Dei and L. Sorace, Dalton Trans., 2007, 5253–5259 RSC.
- A. Witt, F. W. Heinemann, S. Sproules and M. M. Khusniyarov, Chem.–Eur. J., 2014, 20, 11149–11162 CrossRef CAS PubMed.
- X.-Y. Chen, R.-J. Wei, L.-S. Zheng and J. Tao, Inorg. Chem., 2014, 53, 13212–13219 CrossRef CAS PubMed.
- A. Tashiro, S. Kanegawa, O. Sato and Y. Teki, Polyhedron, 2013, 66, 167–170 CrossRef CAS.
- Y. Teki, M. Shirokoshi, S. Kanegawa and O. Sato, Eur. J. Inorg. Chem., 2011, 2, 3761–3767 Search PubMed.
- O. Sato, J. Photochem. Photobiol., C, 2004, 5, 203–223 CrossRef CAS.
- A. Beni, A. Dei, D. A. Shultz and L. Sorace, Chem. Phys. Lett., 2006, 428, 400–404 CrossRef CAS.
- C. Carbonera, A. Dei, J. F. Létard, C. Sangregorio and L. Sorace, Angew. Chem., Int. Ed., 2004, 43, 3136–3138 CrossRef CAS PubMed.
- D. M. Adams and D. N. Hendrickson, J. Am. Chem. Soc., 1996, 118, 11515–11528 CrossRef CAS.
- O.-S. Jung, D. H. Jo, Y.-A. Lee, H. K. Chae and Y. S. Sohn, Bull. Chem. Soc. Jpn., 1996, 69, 2211–2214 CrossRef CAS.
- W. Armarego and C. Chai, Purification of Laboratory Chemicals, Butterworth-Heinemann, 2012, p. 1024 Search PubMed.
- Y. Mulyana, G. Poneti, B. Moubaraki, K. S. Murray, B. F. Abrahams, L. Sorace and C. Boskovic, Dalton Trans., 2010, 4757–4767 RSC.
- E. Evangelio, C. Rodriguez-Blanco, Y. Coppel, D. N. Hendrickson, J. P. Sutter, J. Campo and D. Ruiz-Molina, Solid State Sci., 2009, 11, 793–800 CrossRef CAS.
- J.-F. Létard, L. Capes, G. Chastanet, N. Moliner, S. Létard, J.-A. Real and O. Kahn, Chem. Phys. Lett., 1999, 313, 115–120 CrossRef.
- T. Tezgerevska, K. G. Alley and C. Boskovic, Coord. Chem. Rev., 2014, 268, 23–40 CrossRef CAS.
- R. V. Chamberlin, G. Mozurkewich and R. Orbach, Phys. Rev. Lett., 1984, 52, 867–870 CrossRef CAS.
- C. Sangregorio, T. Ohm, C. Paulsen, R. Sessoli and D. Gatteschi, Phys. Rev. Lett., 1997, 78, 4645–4648 CrossRef CAS.
- C. Carbonera, A. Dei, C. Sangregorio and J.-F. Létard, Chem. Phys. Lett., 2004, 396, 198–201 CrossRef CAS.
- C. Carbonera, A. Dei, J.-F. Ltard, C. Sangregorio and L. Sorace, Inorg. Chim. Acta, 2007, 360, 3825–3828 CrossRef CAS.
- A. Cui, K. Takahashi, A. Fujishima and O. Sato, J. Photochem. Photobiol., A, 2004, 167, 69–73 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5fd00088b |
| This journal is © The Royal Society of Chemistry 2015 |