Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural investigation of Na3NpO4 and Na3PuO4 using X-ray diffraction and 237Np Mössbauer spectroscopy†
A. L.
Smith
*ab,
P. E.
Raison
*a,
A.
Hen
a,
D.
Bykov
a,
E.
Colineau
a,
J.-P.
Sanchez
cd,
R. J. M.
Konings
a and
A. K.
Cheetham
b
aEuropean Commission, Joint Research Centre, Institute for Transuranium Elements, P.O. Box 2340, D-76125 Karlsruhe, Germany. E-mail: als77@cantab.net; philippe.raison@ec.europa.eu
bDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge, CB3 0FS, UK
cCEA, INAC-SPSMS, FR-38000, Grenoble, France
dUniversité de Grenoble Alpes, INAC-SPSMS, FR-38000, Grenoble, France
First published on 8th September 2015
Abstract
α-Na3NpO4 and α-Na3PuO4 exhibit an orthorhombic structure (Z = 8), in space group Fmmm, with lattice parameters a = 13.352(2) Å, b = 9.629(2) Å, and c = 6.673(2) Å for the neptunium compound, and a = 13.302(2) Å, b = 9.634(2) Å, and c = 6.651(2) Å for the plutonium analogue. The corresponding structure has been solved ab initio as no structural analogue could be found in the literature. The pentavalent state of neptunium has moreover been confirmed by 237Np Mössbauer spectroscopy, and the local structural properties inferred from the X-ray Rietveld refinement have been related to the fitted quadrupole coupling constant and asymmetry parameters. The existence of a low temperature metastable m phase of Na3NpO4 and Na3PuO4, of the NaCl type, has also been suggested.
1. Introduction
The long-term storage of high-level radioactive waste, especially of the long lived actinides (Np, Am, Cm) generated during the irradiation process in conventional nuclear reactors, is a subject of primary concern for the nuclear industry with respect to the public. One solution to reduce the waste's radiotoxic inventory is to recover the long-lived isotopes from the spent fuel and re-irradiate them in a fast reactor to transmute them into less radioactive elements with shorter half-lives.1,2 Operating in a closed fuel cycle and with a fast neutron spectrum, Sodium-cooled Fast Reactors (SFR) are currently considered as one of the most advanced options in terms of management of the actinides.3,4 Moreover, SFRs present appealing advantages over the current second generation reactors, especially because of their improved energy efficiency. The sodium metallic coolant allows a significant margin to overheating due to its high heat capacity, and its boiling point (1156 K) which is much higher than the reactor's operating temperature.A safety concern for these reactors comes, however, from the potential interaction of the (U, Pu)O2 mixed oxide fuel with the metallic sodium coolant in the event of a breach of the stainless steel cladding. The reaction between sodium and urania–plutonia solid solution leads to the formation of Na3(U, Pu)O4, a compound with lower density and thermal conductivity than the mixed oxide fuel.5–7 The introduction of minor actinides into the fuel, i.e. (U, Np, Pu, Am)O2, will introduce a much more complex chemistry for which many data are still missing.
Keller and coworkers pioneered the study of the interaction between minor actinides (Np, Am) and alkali metals.8,9 As part of our program of research, we have recently revisited the structural properties of the Na–Np–O10,11 and Na–Pu–O systems,12 and confirmed the formation of the hexavalent and heptavalent compounds Na2NpO4, Na4AnO5, Na2An2O7, and Na5AnO6 (An = Np, Pu). The present study focuses on the pentavalent composition Na3AnO4, as this product is more likely to form under the oxygen potential conditions of the reactor.
Keller et al. reported in 1965 the existence of Na3AnO4 (An = Np, Pu, Am). They suggested Na3AmO4 had a pure NaCl type of structure with cell parameter a = 4.75 Å, while Na3NpO4 and Na3PuO4 adopted a NaCl-type superlattice structure.9,13,14 The cell parameters for Na3PuO4 were reported as a = 4.88 Å,6,15 and a = 4.86 Å.16 In 1989, Pillon suggested for the same compound indexing on the basis of a rhombohedral lattice with a = 4.678 Å and α = 60.40°,17 as well as the existence of two phase transitions for this compound at 623 K and 1048 K, respectively. The literature is quite confusing with respect to the Na3AnO4 (An = Np, Pu, Am) composition, and a detailed crystal structure analysis is lacking. The ionic radii of pentavalent uranium, neptunium, and plutonium being very close (0.76, 0.75 and 0.74 Å, respectively, for 6-fold coordination),18 one could expect the corresponding Na3AnO4 structures to be similar. There are some indications in the reported data that this is not the case, however.
In the present work, the structures of α-Na3NpO4 and α-Na3PuO4 have been solved using room temperature X-ray diffraction. The pentavalent state of the neptunium cation has moreover been confirmed by 237Np Mössbauer spectroscopy, and the local structural properties inferred from the X-ray refinement have been related to the fitted Mössbauer parameters, i.e. quadrupole coupling constant and asymmetry parameters. The existence of a low temperature cubic phase of Na3NpO4 and Na3PuO4 has finally been revealed.
2. Experimental section
2.1. Raw materials and solid state synthesis
α-Na3NpO4 was prepared by grinding together neptunium dioxide (237NpO2 from ORNL, Oak Ridge) with sodium oxide (Na2O 82.1% + Na2O2 14.8%, ABCR GmbH & Co, i.e. Na2O1.14(1)) in a (NpO2 : Na2O1.14(1)) = (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) ratio in an argon filled dry glove box. Sodium oxide was carefully stored in the dry atmosphere of the glove box because of its hygroscopic nature. The (NpO2 : Na2O1.14(1)) mixture was introduced into a stainless steel container that was tightly closed under the purified argon atmosphere of the glove box, and heated in a tubular furnace at 1123 K for 24 hours. No secondary phases were detected by X-ray diffraction, and the prepared sample was subsequently used for the Mössbauer measurement (section 4).
2.4) ratio in an argon filled dry glove box. Sodium oxide was carefully stored in the dry atmosphere of the glove box because of its hygroscopic nature. The (NpO2 : Na2O1.14(1)) mixture was introduced into a stainless steel container that was tightly closed under the purified argon atmosphere of the glove box, and heated in a tubular furnace at 1123 K for 24 hours. No secondary phases were detected by X-ray diffraction, and the prepared sample was subsequently used for the Mössbauer measurement (section 4).
The existence of a metastable cubic disordered phase of Na3NpO4 (m phase) was also investigated by mixing neptunium dioxide with sodium oxide in the same (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) ratio, and heating the mixture for 12 hours under argon flow at successively 673 and 873 K. The latter material was also measured by Mössbauer spectroscopy, as detailed in section 5.
2.4) ratio, and heating the mixture for 12 hours under argon flow at successively 673 and 873 K. The latter material was also measured by Mössbauer spectroscopy, as detailed in section 5.
The application of the aforementioned synthesis route to plutonium dioxide (239PuO2 from ITU-JRC stocks) did not lead to the formation of α-Na3PuO4, but to Na2PuO3,12 which is a monoclinic phase, in space group C2/c. α-Na3PuO4 was nevertheless obtained by mixing plutonium dioxide with sodium carbonate (Na2CO3 99.95%, Sigma) in a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) ratio, and heating the mixture under a flow of argon in a tubular furnace at 1093 K for 80 hours with intermediate regrinding steps. The α-Na3NpO4 phase was also obtained with this method, but with small admixtures of Na4NpO5 and unreacted NpO2. The latter material was used for the high temperature X-ray diffraction study reported in the ESI.†
2) ratio, and heating the mixture under a flow of argon in a tubular furnace at 1093 K for 80 hours with intermediate regrinding steps. The α-Na3NpO4 phase was also obtained with this method, but with small admixtures of Na4NpO5 and unreacted NpO2. The latter material was used for the high temperature X-ray diffraction study reported in the ESI.†
2.2. Powder X-ray diffraction
The samples were characterized at room temperature by X-ray diffraction using a Bruker D8 X-ray diffractometer mounted in the Bragg–Brentano configuration with a curved Ge monochromator (111), and a ceramic copper tube (40 kV, 40 mA) equipped with a LinxEye position sensitive detector. The data were collected by step scanning in the angle range 10° ≤ 2θ ≤ 120°, with an integration time of about 8 h, a count step of 0.02° (2θ), and a dwell of 5 s per step. Structural analysis was performed by the Rietveld method with the Fullprof2k suite.192.3. Mössbauer spectroscopy
The 237Np Mössbauer spectroscopy measurements were carried out in transmission, using an 241Am metal source with a sinusoidal driving mode. The effect was measured with a photon energy of 59.54 keV. The powder samples, encapsulated in 3 concentric aluminium containers, were probed in the temperature range 4.2–20 K, while the source was kept at a constant temperature of 4.2 K inside an independent chamber in the stainless steel cryostat. The velocity scale was moreover calibrated with respect to NpAl2.3. Structural refinement
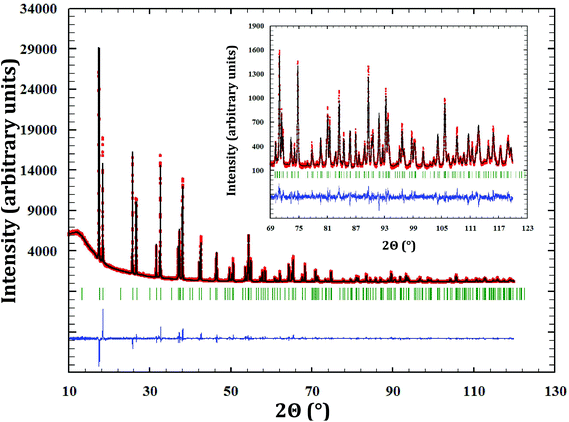
3.1. α-Na3NpO4
A brand new phase, for which there is no report in the literature and no structural analogue, was synthesized by reaction at 1123 K between neptunium dioxide and sodium oxide as described in the Experimental section (Fig. 1).An orthorhombic cell, in space group Fmmm (No. 69), was determined using the program NTREOR implemented in EXPO2013.20 The crystallographic structure was then solved by the heavy-atom method, which consists in determining first the position of the heaviest atoms in the compound (here neptunium). This technique is particularly well suited for compounds containing both heavy atoms and much lighter ones, as in the present case. Based on the volume of the unit cell (858.1 Å3), only eight Na3NpO4 molecules can reasonably fit. Besides, only nine Wyckoff positions are possible for the Fmmm space group: two with a multiplicity of 4, six with a multiplicity of 8, and one with a multiplicity of 16. Accordingly, various combinations were tested, taking into account that only 8 neptunium atoms should be found in the unit cell. Eventually, one neptunium on the Wyckoff position (8g), i.e. (x, 0, 0), reproduced reasonably well the experimental X-ray diffraction pattern with an arbitrary value for the fractional coordinate x = 0.1. The latter value was refined by Rietveld analysis. The positions of the lighter atoms – Na and O – were subsequently determined by 3D Fourier differences (using the program GFourier (Version 04.06) of the Fullprof2k suite19), which show the residual electronic density in the unit cell. The final atomic positions were refined by the Rietveld method.
The cell parameters were determined as a = 13.353(2) Å, b = 9.629(2) Å, and c = 6.673(2) Å. Refined atomic positions are listed in Table 1.
| Atom | Ox. state | Wyckoff | x | y | z | B 0 (Å2) |
|---|---|---|---|---|---|---|
| Np | +5 | 8g | 0.1314(1) | 0 | 0 | 0.25(1) |
| Na1 | +1 | 8g | 0.1145(6) | 0.5 | 0 | 0.36(5) |
| Na2 | +1 | 8c | 0 | 0.25 | 0.25 | 0.36(5) |
| Na3 | +1 | 8f | 0.25 | 0.25 | 0.25 | 0.36(5) |
| O1 | −2 | 8i | 0 | 0.5 | 0.251(1) | 1.02(8) |
| O2 | −2 | 8d | 0.25 | 0 | 0.25 | 1.02(8) |
| O3 | −2 | 16o | 0.1300(8) | 0.2146(6) | 0 | 1.02(8) |
Na3NpO4 is suggested to form according to the probable reaction (1). The sodium peroxide impurity present in the commercial sodium oxide starting material is acting as an oxidizing agent allowing the formation of this new pentavalent phase.
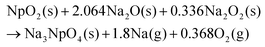 | (1) |
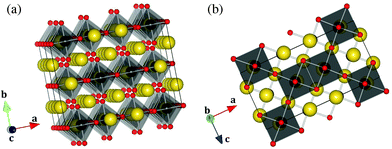
The structure is made of distorted NpO6 octahedra with minimum and maximum bond lengths 2.066(6) and 2.417(6) Å, respectively (Table 2). The O(3)–Np–O(3) bond, pointing along the b direction, is not strictly linear, but has an angle of 179.0°, while the O(1)–Np–O(2) bonds in the equatorial plane form angles of 177.0°. The distortion is also pronounced in the NaO6 octahedra (from 2.267(8) to 2.756(6) Å). As shown in Fig. 2, the NpO6 octahedra are connected to each other via one edge and two corners in the (ac) plane, and form layers perpendicular to the b direction. The Na1 atoms are located within the layers, while the Na2 and Na3 atoms are located in between the layers and bind them together. Na2 and Na3 alternate in rows along the c direction.
| Bond | N | Bond length (Å) | |
|---|---|---|---|
| α-Na3NpO4 | α-Na3PuO4 | ||
| An–O(1) | 2 | 2.417(6) | 2.41(2) |
| An–O(2) | 2 | 2.300(6) | 2.29(1) |
| An–O(3) | 2 | 2.066(6) | 2.29(2) |
| Na(1)–O(1) | 2 | 2.267(8) | 2.25(3) |
| Na(1)–O(2) | 2 | 2.461(6) | 2.46(2) |
| Na(1)–O(3) | 2 | 2.756(6) | 2.60(2) |
| Na(2)–O(1) | 2 | 2.407(2) | 2.41(1) |
| Na(2)–O(3) | 4 | 2.432(8) | 2.61(2) |
| Na(3)–O(2) | 2 | 2.407(2) | 2.41(1) |
| Na(3)–O(3) | 4 | 2.338(7) | 2.12(1) |
| Bond | Bond angle (°) | |
|---|---|---|
| α-Na3NpO4 | α-Na3PuO4 | |
| O(1)–An–O(2) | 177.0(1) | 177.0(2) |
| O(3)–An–O(3) | 179.0(3) | 166.8(4) |
| O(1)–An–O(1) | 87.0(1) | 87.1(3) |
| O(2)–An–O(2) | 93.0(1) | 93.1(2) |
| O(3)–An–O(1) | 89.6(2) | 94.8(3) |
| O(3)–An–O(2) | 90.4(1) | 85.2(2) |
3.2. α-Na3PuO4
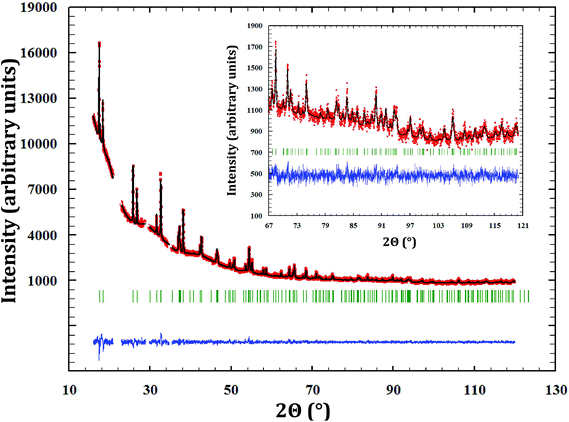
α-Na3PuO4 is isostructural with its neptunium analogue. The refinement yielded in this case lattice parameters as a = 13.302(2) Å, b = 9.634(2) Å, and c = 6.651(2) Å. The unit cell volumes of the neptunium (858.1 Å3) and plutonium (852.3 Å3) compounds are consistent with the ionic radii of Np5+ and Pu5+ (0.75 and 0.74 Å, respectively18). The refined atomic positions are presented in Table 3. The corresponding distances and angles are listed in Table 2. The X-ray diffraction pattern is shown in Fig. 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a = 26.6, Rexp = 21.42, χ2 = 1.54
a = 26.6, Rexp = 21.42, χ2 = 1.54
| Atom | Ox. state | Wyckoff | x | y | z | B 0 (Å2) |
|---|---|---|---|---|---|---|
| a The rather large value of Rwp is related to the experimental constraints for the plutonium compound: small amount of material, and encapsulation for the X-ray measurement in glue resulting in a high background level and rather poor signal to background ratio. | ||||||
| Pu | +5 | 8g | 0.1315(3) | 0 | 0 | 0.41(1) |
| Na1 | +1 | 8g | 0.114(2) | 0.5 | 0 | 0.40(5) |
| Na2 | +1 | 8c | 0 | 0.25 | 0.25 | 0.40(5) |
| Na3 | +1 | 8f | 0.25 | 0.25 | 0.25 | 0.40(5) |
| O1 | −2 | 8i | 0 | 0.5 | 0.250(4) | 1.08(8) |
| O2 | −2 | 8d | 0.25 | 0 | 0.25 | 1.08(8) |
| O3 | −2 | 16o | 0.151(2) | 0.236(2) | 0 | 1.08(8) |
3.3. The Na3AnO4 structure along the series of the actinide elements (An = U, Np, Pu)
The mean Np–O distance, obtained after refinement of the Na3NpO4 structure, is consistent with the ones obtained for hexavalent (Na2NpO4, Na4NpO5, Na2Np2O7) and heptavalent (Na5NpO6) sodium neptunate compositions,10 where neptunium is also 6-fold coordinated. As shown in Fig. 4, this distance decreases when the valence state of neptunium increases. The same trend is verified for the 6-fold coordinated PuO6 octahedra. The mean Pu–O distance in Na3PuO4 (2.33(2) Å) is moreover consistent with the one reported for pentavalent Na5PuO5 (2.28(1) Å).12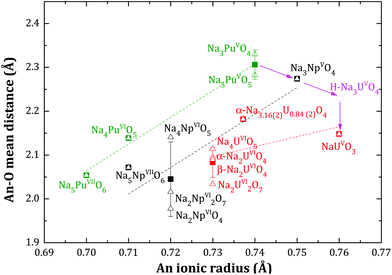 | ||
Fig. 4 Mean An–O distances (An = U, Np, Pu) in the AnO6 octahedra of various sodium uranate, neptunate, and plutonate compositions as a function of the ionic radius of the actinide ion after the data of Shannon18 ( , Δ, , Δ,  ). The squares ( ). The squares ( , ■, , ■,  ) – and their associated error bars – show the average distance for a given actinide, and a given valence state. The dashed lines are linear trends of average distances for a given actinide. H-Na3UO4 is an hypothetical phase, orthorhombic, in space group Fmmm. ) – and their associated error bars – show the average distance for a given actinide, and a given valence state. The dashed lines are linear trends of average distances for a given actinide. H-Na3UO4 is an hypothetical phase, orthorhombic, in space group Fmmm. | ||
Although the ionic radii of Np5+ and Pu5+ are very close (0.75 and 0.74 Å, respectively, in 6-fold coordination) to that of U5+ (0.76 Å),18 the Na3NpO4 and Na3PuO4 structures differ from the corresponding phase of uranium. The structure of the trisodium uranate Na3(U1−x,Nax)O4 (0 < x < 0.16(2)) was recently investigated21 and shown to exhibit three polymorphs: a low temperature metastable NaCl type of structure (m phase), a stable α monoclinic phase, in space group P21, and a high temperature β cubic phase, in space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.21 It was also shown that the α phase can accommodate excess sodium on the uranium site up to the composition Na3(U0.84(2)Na0.16(2))O4. The charge balance is then realized by the uranium cation, which adopts a mixed valence state with (76 ± 12%) U(VI) and (24 ± 12%) U(V).
m.21 It was also shown that the α phase can accommodate excess sodium on the uranium site up to the composition Na3(U0.84(2)Na0.16(2))O4. The charge balance is then realized by the uranium cation, which adopts a mixed valence state with (76 ± 12%) U(VI) and (24 ± 12%) U(V).
Following the trend shown in Fig. 4, one would expect an orthorhombic α-Na3U(V)O4 phase with a mean U–O distance around 2.23 Å., i.e. slightly higher than the mean U–O distance in pentavalent NaU(V)O3 compound (2.15 Å). However, we did not succeed in synthesizing this phase either with sodium oxide or sodium carbonate, suggesting that orthorhombic Na3UO4 cannot form as a stable phase. The compound adopts instead a monoclinic or cubic phase, with mixed valence state composition Na3(U1−x,Nax)O4 (0 < x < 0.16(2)), and a mean U–O distance around 2.18(1) Å.
4. Mössbauer spectroscopy studies of α-Na3NpO4
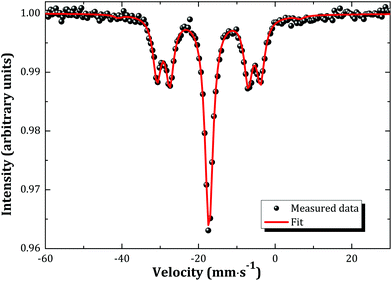
The Mössbauer spectrum of α-Na3NpO4 recorded at 4.2 K is shown in Fig. 5. It consists of a single quadrupolar split pattern centred at −17.3 mm s−1, which corresponds to an isomer shift of δIS = −30.9(3) mm s−1 relative to the standard NpAl2 absorber. The latter value lies in the range −11.0 < δIS < −37.5 mm s−1, which confirms the Np(V) charge state, corresponding to a [Rn]5f2 electronic configuration, as displayed in the correlation diagram in Fig. 6. The pure Np(V) valence state derived herein is a proof that a mechanism of incorporation of Na on the neptunium site (and charge compensation by the neptunium cation) does not occur, by contrast with the uranium phase.21 It is suggested that this is also the case for the plutonium phase, but X-ray Absorption Near-Edge Structure spectroscopy or 23Na Magic Angle Spinning Nuclear Magnetic Resonance measurements would be needed to confirm this hypothesis.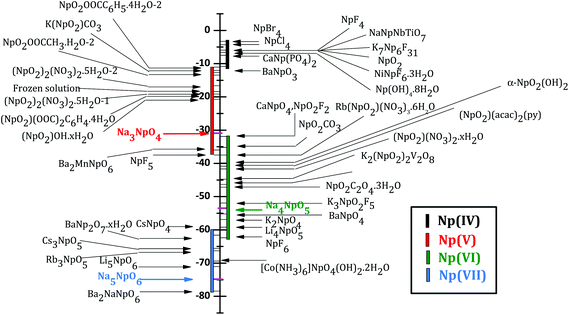 | ||
| Fig. 6 Isomer shifts versus NpAl2 of Np(IV), Np(V), Np(VI), and Np(VII) compounds after (ref. 22). The present result for Na3NpO4 is shown in red together with Na4NpO5 (green) and Na5NpO6 (blue) reported in (ref. 11). | ||
The spectral shape remains the same between 4.2 and 20 K, except for a slight decrease in overall effect with increasing temperature, which is attributed to the temperature dependance of the Lamb–Mössbauer factor. The occurrence of a phase transition (either crystallographic or magnetic) within the probed temperature range is therefore excluded.
The existence of a quadrupole coupling constant |e2qQ| = 48.6(3) mm s−1, and non vanishing asymmetry parameter η = 0.68(3), indicates a lower symmetry than Oh in α-Na3NpO4. These results are in good agreement with the local symmetry around the neptunium ion as determined by X-ray diffraction, i.e. rather strongly distorted NpO6 octahedra. It is interesting to compare the quadrupole coupling constant measured here with the values for other pentavalent compounds, in particular (NpO2)2SO4, 2H2O (|e2qQ| = 82.4(1.0) mm s−1), and Na4(NpO2)2C12O12·8H2O (|e2qQ| = 94.0(1.0) mm s−1).23 Those compounds both present (NpO2)+ neptunyl type of ions, i.e. with two close oxygen neighbours in the axial direction, which leads to much higher values of the quadrupole coupling constants (around 100 mm s−1). The same observation was made for hexavalent phases.23
5. A low temperature metastable NaCl type of structure
Literature dating back to the sixties and seventies suggests a NaCl type of structure for Na3AnO4 (An = U, Np, Pu, Am).9,13,14,24 The existence of such a phase for neptunium was re-investigated in the present work by reaction between neptunium dioxide and sodium oxide at low temperatures (673 K) under argon flow. This led to the formation of a poorly crystallized cubic product “X”, with cell parameter a = 4.746(5) Å, similar to the one reported for Na3AmO4 (a = 4.75 Å).9Mössbauer spectroscopy measurements were performed so as to determine the exact oxidation state and chemical composition of product “X”. Under the assumption that there are no magnetic phases in “X”, the measured Mössbauer spectrum (Fig. 7) revealed two distinct sites (A and B) with isomer shifts (with respect to NpAl2 standard) at δIS,A = −32.2(3) mm s−1 (47.5% for A) and δIS,B = −52.7(3) mm s−1 (52.5% for B), corresponding to Np(V) and Np(VI), respectively. The existence of two valence states shows there must be oxygen vacancies within the lattice and explains the observed disordered structure of product “X”. The local symmetry is therefore not perfectly cubic. The shape of the experimental spectrum is adequately described by taking into account that the quadrupolar interactions acting at both A and B sites are distributed. Symmetric distributions of the quadrupole coupling constants around their mean values |e2qQ|A = 67.2(5) mm s−1 and |e2qQ|B = 36.2(5) mm s−1 are assumed. The standard deviations of the distributions are found to be σA,B = 18.7 mm s−1.
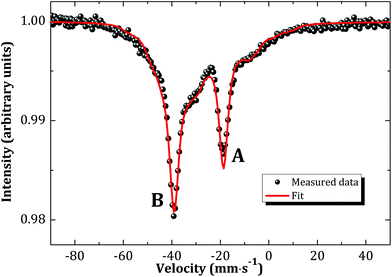 | ||
| Fig. 7 Mössbauer spectrum of the cubic phase “X” recorded at 4.2 K and fitted to the model (see text). | ||
Under those synthesis conditions, one can reasonably expect to form tetravalent (Na2NpO3![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Na2/3Np1/3O), pentavalent (Na3NpO4
Na2/3Np1/3O), pentavalent (Na3NpO4![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Na3/4Np1/4O), or hexavalent (Na4NpO5
Na3/4Np1/4O), or hexavalent (Na4NpO5![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Na4/5Np1/5O) compounds, which can be written with the more general formula Na1−xNpxO with x = {1/5–1/3}. The synthesized product “X”, with an average valence of 5.525, corresponds to the chemical composition Na0.779Np0.221O or Na3.525NpO4.525, according to the ratio of the relative areas of the sub-spectra of Np(V) and Np(VI). There again, the sodium peroxide impurity present in the starting synthesis materials is acting as an oxidizing agent during the solid state reaction. The final product corresponds either to a solid solution between the two end-members m-Na3NpO4 and m-Na4NpO5, or to a mixture of these same phases whose lattice parameters are too close to be distinguished in the present X-ray diffraction pattern showing very broad X-ray reflections. The existence of the m-Na4NpO5 cubic phase was reported in the literature with a cell parameter as 4.739 Å,8 but is subject of controversy.14,17 A more detailed study of the possible reactions products forming at low temperatures (T < 873 K) depending on the oxygen potential conditions would be needed to clarify this point, and discard one of these two hypotheses.
Na4/5Np1/5O) compounds, which can be written with the more general formula Na1−xNpxO with x = {1/5–1/3}. The synthesized product “X”, with an average valence of 5.525, corresponds to the chemical composition Na0.779Np0.221O or Na3.525NpO4.525, according to the ratio of the relative areas of the sub-spectra of Np(V) and Np(VI). There again, the sodium peroxide impurity present in the starting synthesis materials is acting as an oxidizing agent during the solid state reaction. The final product corresponds either to a solid solution between the two end-members m-Na3NpO4 and m-Na4NpO5, or to a mixture of these same phases whose lattice parameters are too close to be distinguished in the present X-ray diffraction pattern showing very broad X-ray reflections. The existence of the m-Na4NpO5 cubic phase was reported in the literature with a cell parameter as 4.739 Å,8 but is subject of controversy.14,17 A more detailed study of the possible reactions products forming at low temperatures (T < 873 K) depending on the oxygen potential conditions would be needed to clarify this point, and discard one of these two hypotheses.
The compound “X” was furthermore heated up to 1473 K under argon flow. The X-ray analysis performed on the sample after cooling down to room temperature revealed β-Na4NpO5 as the major phase, and α-Na3NpO4 as a secondary phase. Supposing that m-Na4NpO5 and m-Na3NpO4 form a solid solution at low temperatures, the heating treatment up to 1473 K leads to phase separation (demixing) into the two equilibrium phases.
Bykov et al., who recently revisited the Na–Pu–O system, performed an exhaustive study of the various reaction products forming at low temperatures by reaction between plutonium dioxide and sodium oxide.12 The authors reported the formation, after heating at 673 K for 24 hours under oxygen flow, of a cubic phase of cell parameter (a = 4.73 Å) (phase c.p. I), when mixing PuO2 and Na2O in (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8), (1
1.8), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6), and (1
3.6), and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8) ratios. Under argon flow, the mixing of PuO2 and Na2O in (1
4.8) ratios. Under argon flow, the mixing of PuO2 and Na2O in (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2), (1
1.2), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8), (1
1.8), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4), and (1
2.4), and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6) ratios, led to the formation of a cubic phase of cell parameter (a = 4.77 Å) (phase c.p. II). Slow heating of the phase c.p. II up to 1073 K under argon flow moreover led to the formation of α-Na3PuO4 together with small unidentified impurities. It is suggested that the phase c.p. I corresponds to Pu(VI), while c.p. II is closer to Pu(V). It could be either pure Pu(V), or a mixed valence state compound Pu(V)/Pu(VI) such as product “X”. This is consistent with the smaller ionic radius of Pu6+ (0.71 Å), compared to Pu5+ (0.74 Å) according to Shannon's tabulated data.18
3.6) ratios, led to the formation of a cubic phase of cell parameter (a = 4.77 Å) (phase c.p. II). Slow heating of the phase c.p. II up to 1073 K under argon flow moreover led to the formation of α-Na3PuO4 together with small unidentified impurities. It is suggested that the phase c.p. I corresponds to Pu(VI), while c.p. II is closer to Pu(V). It could be either pure Pu(V), or a mixed valence state compound Pu(V)/Pu(VI) such as product “X”. This is consistent with the smaller ionic radius of Pu6+ (0.71 Å), compared to Pu5+ (0.74 Å) according to Shannon's tabulated data.18
6. Conclusion
The structures of Na3NpO4 and Na3PuO4 have been determined in the present work for the first time. The compounds were shown to exhibit a completely disordered NaCl type of structure at low temperatures (T < 873 K), corresponding to a metastable m phase, that transforms to the equilibrium ordered α phase (with orthorhombic symmetry, in space group Fmmm), when heated at higher temperatures (T > 1093 K). The α structures have been refined by the Rietveld method. The pentavalent state of neptunium has been confirmed from the isomer shift value obtained by Mössbauer spectroscopy δIS(Na3NpO4) = −30.9(3) mm s−1. The quadrupole coupling constant, |e2qQ| = 48.6(3) mm s−1, and rather high asymmetry parameter, η = 0.68(3), have been related to the distortion of the NpO6 octahedra in this structure, which show Np–O bond lengths between 2.066(6) and 2.417(6) Å, and non linear O(3)–Np–O(3) (179.0°) and O(1)–Np–O(2) (177.0°) bonds. Interestingly, Na3NpO4 and Na3PuO4 do not adopt the same monoclinic structure as Na3(U1−x,Nax)O4 (0 < x < 0.16(2)) despite having very close ionic radii. It is suggested that orthorhombic Na3UO4 cannot form as a stable phase.Acknowledgements
The authors would like to express their gratitude to D. Bouëxière and G. Pagliosa for the collection of room temperature and high temperature X-ray diffraction data. They also thank the 7th Framework Program of the European Commission, and the Joint Advanced Severe Accidents Modelling and Integration for Na-cooled neutron reactors (JASMIN) programme (No. 295803 in FP7). ALS acknowledges the European Commission and the Ras al Khaimah Centre for Advanced Materials for funding her PhD studentship.References
- L. Koch, Minor actinide transmutation - A waste management option, J. Less-Common Met., 1986, 122, 371–382 CrossRef CAS.
- C. T. Walker and G. Nicolaou, Transmutation of neptunium and americium in a fast neutron flux: EPMA results and KORIGEN predictions for the superfact fuels, J. Nucl. Mater., 1995, 218, 129–138 CrossRef CAS.
- GIF, Annual report 2013, Generation IV International Forum, Tech. rep., 2013.
- GIF, Technology Roadmap Update for Generation IV Nuclear Energy Systems, Tech. rep., 2014.
- P. E. Blackburn and W. K. Hubbard, Proceedings of Conference on Fast Reactor Fuel Element Technology, Hindsdale, Amer. Nucl. Soc., Hinsdale, Illinois, 1972, p. 479 Search PubMed.
- M. Housseau, G. Dean and F. Perret, Behaviour and Chemical State of Irradiated Ceramic Fuels, in Panel Proceedings Series, IAEA, Vienna, 1974, p. 349 Search PubMed.
- M. Housseau, G. Dean, J.-P. Marcon and J. F. Marin, Report CEA-N-1588 (Commissariat à l’énergie atomique et aux énergies alternatives), 1973.
- C. Keller, L. Koch and K. H. Walter, Die Reaktion der Oxide der Transurane mit Alkalioxiden-I Ternäre Oxide der Sechswertigen Transurane mit Lithium und Natrium, J. Inorg. Nucl. Chem., 1965, 27, 1205–1223 CrossRef CAS.
- C. Keller, L. Koch and K. H. Walter, Die reaktion der Transuranoxide mit Alkalioxiden-II Ternäre oxide der fünfwertigen Transurane und des Proctactiniums mit Lithium und Natrium, J. Inorg. Nucl. Chem., 1965, 27, 1225–1232 CrossRef CAS.
- A. L. Smith, P. E. Raison and R. J. M. Konings, Synthesis and crystal structure characterisation of sodium neptunate compounds, J. Nucl. Mater., 2011, 413, 114–121 CrossRef CAS.
- A. L. Smith, A. Hen, P. E. Raison, E. Colineau, J.-C. Griveau, N. Magnani, J. P. Sanchez, R. J. M. Konings, R. Caciuffo and A. K. Cheetham, X-ray diffraction, Mössbauer spectroscopy, magnetic susceptibility, and specific heat investigations of Na4NpO5 and Na5NpO6, Inorg. Chem., 2015, 54, 4556–4564 CrossRef CAS PubMed.
- D. Bykov, P. Raison, R. J. M. Konings, C. Apostolidis and M. Orlova, Synthesis and crystal structure investigations of ternary oxides in the Na-Pu-O system, J. Nucl. Mater., 2015, 457, 54–62 CrossRef CAS.
- C. Keller, MTP International Review of Science, in Inorg. Chem., ser. 1, ed. K. Bagnall, Butterworths, London, 1972, vol. 7, p. 479 Search PubMed.
- L. R. Morss, Actinides-1981, Pergamon, Oxford, 1982, pp. 381–407 Search PubMed.
- M. A. Mignanelli and P. E. Potter, The reactions between sodium and plutonia, urania-plutonia and urania-plutonia containing fission products simulants, J. Nucl. Mater., 1984, 125, 182–201 CrossRef CAS.
- M. A. Mignanelli and P. E. Potter, The reactions of sodium with urania, plutonia and their solid solutions, J. Nucl. Mater., 1985, 130, 289–297 CrossRef CAS.
- S. Pillon, Etude des diagrammes de phases U-O-Na, Pu-O-Na et U,Pu-O-Na, Ph.D. thesis, Univ. Du Languedoc, 1989 Search PubMed.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chaleogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751–767 CrossRef.
- J. Rodriguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Physica B, 1993, 192, 55–69 CrossRef CAS.
- A. Altomare, C. Cuocci, C. Giacovazzo, A. Moliterni, R. Rizzi, N. Corriero and A. Falcicchio, EXPO2013: a kit of tools for phasing crystal structures from powder data, J. Appl. Crystallogr., 2013, 46, 1231–1235 CrossRef CAS.
- A. L. Smith, P. E. Raison, L. Martel, D. Prieur, T. Charpentier, G. Wallez, E. Suard, A. C. Scheinost, C. Hennig, P. Martin, K. O. Kvashnina, A. K. Cheetham and R. J. M. Konings, A new look at the structural properties of trisodium uranate Na3UO4, Inorg. Chem., 2015, 54, 3552–3561 CrossRef CAS PubMed.
- Z. Yoshida, S. G. Johnson, T. Kimura and J. R. Krsul, Chapter 6: Neptunium, in The Chemistry of the Actinide and Transactinide Elements, ed. L. R. Morss, N. Edelstein, J. Fuger, J. J. Katz, 2006, pp. 699–812 Search PubMed.
- J. Jové, J. Proust, M. Pagès and P. Pyykkö, Mössbauer spectroscopy as a nuclear probe for solid state transuranium chemistry, J. Alloys Compd., 1991, 177, 285–310 CrossRef.
- R. Scholder and H. Gläser, Über Lithium- und Natriumuranate(V) und über strukturelle Beziehungen zwischen den Verbindungstypen Li7AO6 und Li8AO6., Z. Anorg. Allg. Chem., 1964, 327, 15–27 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic files in CIF format. Study of the thermal expansion of α-Na3NpO4 structure solution. See DOI: 10.1039/c5dt02168e |
| This journal is © The Royal Society of Chemistry 2015 |