Open Access Article
Open Access ArticleTwist grain boundary (TGB) states of chiral liquid crystalline bent-core mesogens†
Hale
Ocak
*ab,
Belkız
Bilgin-Eran
b,
Dilek
Güzeller
b,
Marko
Prehm
a and
Carsten
Tschierske
*a
aInstitute of Chemistry, Organic Chemistry, Martin-Luther University Halle-Wittenberg, Kurt-Mothes Str. 2, 06120 Halle, Germany. E-mail: carsten.tschierske@chemie.uni-halle.de; Fax: +49 345 5527346
bDepartment of Chemistry, Yildiz Technical University, Davutpasa Yerlesim Birimi, TR-34220, Esenler, Istanbul, Turkey. E-mail: ocak_hale@hotmail.com; Fax: +90 212 3834134
First published on 24th March 2015
Abstract
4-Cyanoresorcinol derived bent-core molecules with a chiral (S)-2-methylbutoxy chain form liquid crystalline phases with TGBA- and TGBC-like structures at the transition from cybotactic nematic via SmA to SmC phases.
Chirality has huge effects on molecular self-assembly.1 Soft matter, especially liquid crystalline (LC) phases,2,3 can easily be affected by chirality leading to helical superstructures.4 Molecules with high helical twisting power‡ can even give rise to the frustration of the fundamental structures of LC self-assembly, thus providing new superstructures with a higher level of complexity3 and new emerging properties. Examples are the three-dimensional lattices of defects in the blue phases which are of importance as photonic band gap materials and represent candidates for new generations of extremely fast switching electro-optical devices,5 twist grain boundary (TGB) phases,6 representing analogues of the Abrikosov flux phase of type-II superconductors7 and several other complex structures.5,8
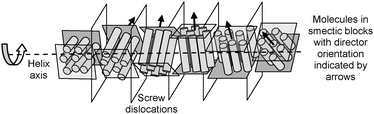
TGB phases, formed by chiral rod-like mesogens, represent helical superstructures with a helix axis parallel to the layer planes thus disrupting the layers to give blocks separated by screw dislocations (Fig. 1). TGB phases can be divided into TGBA phases with an orthogonal organization of the molecules with respect to the planes of these blocks and several different modes of TGBC phases with a tilted organization of the molecules.6 In the recent two decades bent-core (BC) molecules9 have attracted special attention as new LC materials. This is mainly due to their capability to form LC phases with polar order9 and the spontaneous formation of chiral superstructures though the molecules are achiral.9–12 An interesting point concerns the interaction of these chiral superstructures with the molecular chirality provided by stereogenic units. For example, enhancement of chirality was observed by doping chiral LC phases with achiral bent-core mesogens, which lead to the induction of highly frustrated blue phases with helical organization in all three directions instead of only one in the cholesteric phases.5 It was proposed that “highly chiral” conformations11,13 of the bent aromatic cores lead to a high twisting power.‡ For the same reason broad regions of blue phases were recently found for optically active BCLCs.14
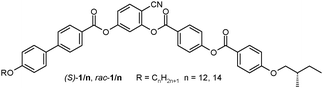
Herein we report the first observation of TGB-like structures for the LC phases of the BC mesogens (S)-1/n (n = 12, 14) involving a 4-cyanoresorcinol derived BC,14b,15 combined with a chiral (S)-2-methylbutoxy group. In order to identify the specific chirality effects, one of the compounds was also synthesized in racemic form (rac-1/12). The synthetic strategy of these BC compounds is outlined in Scheme 1 and the experimental procedures are described in the ESI.†
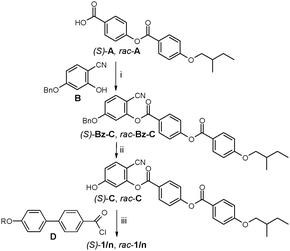 | ||
| Scheme 1 Synthesis of 1/n. Reagents and conditions: (i) DCC, DMAP, CH2Cl2, 20 °C; (ii) H2, Pd/C, THF, 40 °C; (iii) pyridine, CH2Cl2, 20 °C, ESI.† | ||
The observed LC phases, their transition temperatures and associated enthalpies are collated in Table 1. Upon cooling the racemic mixture rac-1/12 from the isotropic liquid (Iso) a nematic phase (N) is formed at first which is then replaced by a lamellar phase (SmA) at T ≤ 96 °C. In the SmA phase there is on average a non-tilted organization of the molecules in layers, whereas at the transition to the SmC phase at T = 88 °C a uniform tilt develops. In calorimetric investigations besides the dominating melting peak only the Iso–N transition could be observed by a small DSC peak (∼1.1 kJ mol−1), whereas there is no measurable enthalpy change for the other LC phase transitions (Fig. S7, ESI†). This indicates that major structural transformations take place at the Iso–N transition and this is in line with a cybotactic structure of the nematic phase, as confirmed by XRD (NcybA, see below).15b It appears that the following phase transitions are continuous and mainly associated with the growth of the cybotactic clusters. All samples can be cooled to about 60 °C without crystallization, so that the monotropic LC phases can also be easily investigated (see Table 1).
| Comp. | T/°C [ΔH/kJ mol−1] |
|---|---|
| a Transition temperatures and enthalpy values (square brackets) determined by DSC (1st heating and cooling, rates 10 K min−1, peak temperatures) or by PM for transitions without visible ΔH; round brackets indicate monotropic (metastable) phases; abbreviations: Cr = crystalline solid; Iso = isotropic liquid; NcybA = cybotactic nematic phase composed of SmA clusters; N*cybA, SmC* = chiral NcybA and SmC phases, respectively; SmA = non-tilted smectic phase; SmC = synclinic tilted smectic phase; TGB = twisted states of the SmA (TGBA) or SmC phases (TGBC) with the helix axis parallel to the layers; for DSCs see Fig. S7 (ESI). | |
| rac-1/12 | Cr 95 [34.7] (SmC 88 [<0.01]) SmA 96 [<0.01] NcybA 108 [1.1] Iso |
| (S)-1/12 | Cr 98 [39.3] (SmC*/TGBC 88 [<0.01] TGBA 96 [<0.01]) N*cybA 108 [1.2] Iso |
| (S)-1/14 | Cr 99 [44.9] (SmC*/TGBC 94 [<0.01]) TGBA 102 [<0.01] N*cybA 109 [1.4] Iso |
The phase transition temperatures of the (S)-enantiomer (S)-1/12 are the same as those found for rac-1/12. For (S)-1/14 a very similar behaviour to those for (S)-1/12 is observed, only the transitions between the LC phases occur at a bit higher temperatures (Table 1). The typical textures observed for the LC phases of (S)-1/n (n = 12, 14) are shown in Fig. 2. A cholesteric oily-streak texture indicates the presence of a helical superstructure in the chiral nematic phases (N*) (Fig. 2a).5 The appearance of the LC phases occurring below N* strongly depends on the alignment conditions. Homeotropic anchoring (layers parallel to the surfaces of the glass substrates) leads to filament textures, as characteristic for TGBA phases16 (Fig. 2b). The filaments rapidly disappear and the textures become uniformly dark, indicating the transition to an optically uniaxial SmA phase. Upon further cooling, a gray and low birefringent texture develops in these homeotropic samples, indicating a transition to a SmC* phase, occurring for (S)-1/12 at about the same temperature as observed for the SmA–SmC transition of the racemate.§ In a cell with planar surface anchoring (layers perpendicular to the substrate surfaces; polyimide-coated ITO cell, 10 μm) the cholesteric oily streak texture changes at the N*–SmA transition to a planar texture composed of differently coloured areas, corresponding to different twist states (Fig. 2c). This texture, which is typical for TGBA phases, is retained upon further cooling (see Fig. S2, ESI†) and at the SmA/SmC phase transition temperature the formation of a grid-like pattern is observed (Fig. 2d), similar to textures of TGBC phases.4,6,17 The texture continuously changes upon further cooling (see Fig. S3, ESI†). Therefore it is not clear if the TGBC structure is retained or a slow transition to a SmC*-phase takes place upon further cooling (therefore it is denoted as SmC*/TGBC in Table 1).
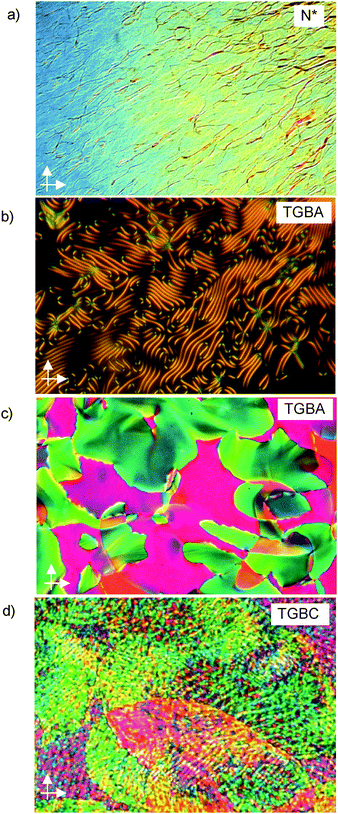 | ||
| Fig. 2 Textures of the LC phases of compounds (S)-1/n between crossed polarisers: (a) oily-streak texture of the N* phase of (S)-1/14 at T = 107 °C; (b) fingerprint texture of (S)-1/14 in the TGBA-state in homeotropic anchoring at T = 96 °C, (c) TGBA-state of (S)-1/12 in planar anchoring (polyimide coated ITO-cell, 10 μm) at T = 92 °C and (d) TGBC-like texture at T = 79 °C; for additional textures see Fig. S2–S5 (ESI†). | ||
XRD investigations were performed for (S)-1/12 and rac-1/12 in thin capillaries at a magnetic field B ∼ 1 T after slow cooling (0.1 K min−1) from the isotropic liquid. In the whole investigated temperature range between 60 and 110 °C the wide angle scattering is diffuse indicating the presence of LC phases without long range order between individual molecules (Fig. 3a and b). In the small angle range there is a diffuse scattering in the N and N* phases, exceeding the wide angle scattering and thus confirming cybotactic nematic phases composed of smectic clusters.15b For rac-1/12 the small angle scattering maximum is on the meridian, indicating that the molecules have no uniform tilt in these domains, i.e. the nematic phases are composed of SmA clusters (NcybA), which is rarely found for BC molecules.15b The intensity of the small angle scattering increases further at the transition to the SmA phase (Fig. 3d). The d-spacings of 4.05–4.10 nm, corresponding to ∼0.85 molecular length (Lmol = 4.8 nm in the most stretched conformation, see Fig. S8, ESI†), are in line with a single layer structure of the cybotactic clusters and layers. The d = f(T) plot for rac-1/12 indicates an increase of d in the N and SmA phases due to an increasing packing density, leading to alkyl chain stretching. A slight decrease of d starts at around the SmA–SmC transition, in line with the onset of uniform tilt at this transition (Fig. 3c). The tilt in the SmC phase is very small (<5°) as estimated for rac-1/12 from the angle between the diffuse wide angle scattering maxima on the equator and the layer reflections on the meridian (XRD tilt). The optical tilt is about 10–15° as estimated from the planar textures of rac-12 (see Fig. S1g and h, ESI†), indicating that the main contribution comes from the tilt of the aromatic cores.
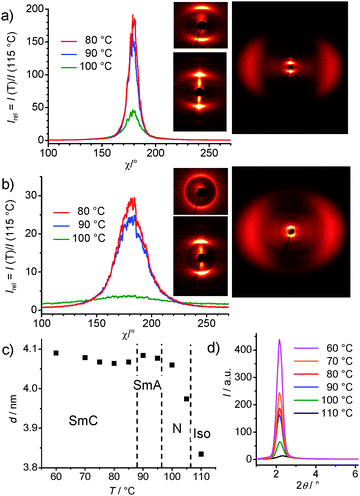 | ||
| Fig. 3 XRD data (a, c, d) of rac-1/12 and (b) of (S)-1/12. (a, b) χ-scans over the small angle scattering depending on T and corresponding 2D XRD patterns of magnetically aligned samples in the N/N* phase at T = 105 °C (top left) and in the tilted smectic phases (bottom and right) at T = 80 °C; (c) T-dependence of the d-spacing and (d) 2θ-scan over the small angle scattering depending on T, see Tables S1, S2 and Fig. S9, S10 (ESI†). | ||
The LC phases of (S)-1/12 (Fig. 3b) have approximately the same d-values as those of rac-1/12. However, in the N* phase the small- and wide-angle scatterings form closed rings, indicating the absence of uniform alignment due to the presence of the helical superstructure. At the transition to TGBA the rings condense to crescent like maxima on the meridian (small angle) and on the equator (wide angle), respectively, in line with the formation of layers. There is a broader angular χ-distribution of the scattering in the smectic phases of (S)-1/12 compared to the sharper peak of rac-1/12 (Fig. 3a and b). However, there is no distribution of the scatterings on a closed ring as it would be expected for TGB phases. So, there seems to be a strong effect of the conditions on the actually observed phase structure. As shown above, planar anchoring stabilizes the TGB helix, whereas homeotropic anchoring has the opposite effect; it stabilizes the layers and tends to remove the TGB helix. Hence, the TGB structures are not considered as “phases”, but as helically deformed states of the underlying smectic phases. The reduced influence of surface anchoring in the capillaries used for XRD, and the alignment of the molecules by the magnetic field, might suppress TGB-helix formation. The broader angular distribution of the scattering of (S)-1/12 might be due to the remaining helical layer distortion or to the less efficient alignment obtained by cooling from the N* phase.
There is no polarization current response under a triangular wave field (PI coated ITO cells 10 μm) in the temperature range of all mesophases for the racemic mixtures as well as for the (S)-enantiomers up to the maximum available value of ±28 V μm−1, indicating the absence of polar order.¶
As an additional interesting point, it should be noted that chiral domains are visible in homeotropic alignment in the range of the SmC phase of the racemic compound rac-1/12. These domains can be identified by slight rotation of the analyzer either clockwise or anti-clockwise out of the precise 90° orientation, which reverses the brightness of these domains (Fig. 4a–c).9,11,13 Rotating the sample between the polarizers does not lead to such a change in brightness, thus excluding tilt director alignment as the origin of this effect (Fig. S6, ESI†). In addition, though domains can also be recognized in the homeotropic SmC* phase of (S)-1/12, in this case there is no visible effect of the orientation of the polarizer (Fig. 4d–f). As there is no polar order in the SmC phase the origin of chirality must be due to the segregation of chiral molecular conformers,13 which is additionally supported by surface interaction.|| This indicates a strong chirality of the molecular conformations and a strong coupling between them. The cybotactic nature of the nematic phase and the absence of clear transition enthalpies at the phase transitions indicate a nearly continuous growth of the coherence length of the smectic clusters throughout the NcybA–SmA–SmC transitions (Fig. 3d, Table S1, ESI†). This indicates that already in the nematic phase the cybotactic clusters are relatively large and continue to increase in the smectic phases. Thus, in the SmA and SmC phases there is a remaining layer distortion providing soft layers which can be easily deformed into a TGB-like superstructure (Fig. 1). Therefore, the weak helical twisting power of the (S)-2-methylbutyl group can provide a sufficiently strong chirality effect such that TGB states are formed at the N*–SmA transition. These can be further stabilized over broader temperature ranges by planar surface anchoring.
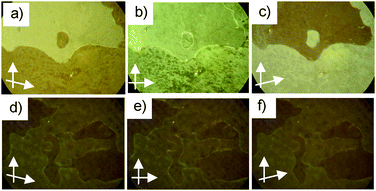 | ||
| Fig. 4 Textures of the homeotropic SmC phases (a–c) of rac-1/12 and (d–f) of (S)-1/12 at T = 75 °C as observed (b,e) between crossed polarizers and between uncrossed polarizers with the analyzer rotated by 5° (a, d) clockwise or (c, f) counter-clockwise; reversal of brightness in the series (a–c) indicates chiral domains with opposite handedness, whereas there is no effect in the series (d–f) (see also Fig. S6, ESI†). | ||
In summary, the first observation of TGB structures in mesophases of bent-core mesogens is reported.** These are formed by molecules with a (S)-2-methylbutoxy stereogenic centre, known to have only weak helical twisting power. However, strong chirality of the molecular conformers, an imperfect layer structure and surface anchoring can stabilize the TGB states.
The work was supported by DFG (Ts 39/24-1); H. O. is grateful to the Alexander von Humboldt Foundation for a research fellowship at Martin Luther University, Halle-Wittenberg; B. B.-E. is grateful to the Alexander von Humboldt Foundation for financial support toward LC research.
Notes and references
- D. B. Amabilino, Chirality at the Nanoscale, Wiley-VCH, Weinheim, 2009 Search PubMed.
- Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. F. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2014 Search PubMed.
- C. Tschierske, Angew. Chem., Int. Ed., 2013, 52, 8828–8878 CrossRef CAS PubMed.
- I. Dierking, Symmetry, 2014, 6, 444–472 CrossRef PubMed.
- Chirality in liquid crystals, ed. H.-S. Kietzerow and C. Bahr, Springer, New York. NY, 2001 Search PubMed.
- (a) J. W. Goodby, M. A. Waugh, S. M. Stein, E. Chin, R. Pindak and J. S. Patel, Nature, 1989, 337, 449–452 CrossRef CAS; (b) J. W. Goodby, Curr. Opin. Colloid Interface Sci., 2002, 7, 326–332 CrossRef CAS; (c) M. Brunet, L. Navailles and N. A. Clark, Eur. Phys. J. E: Soft Matter Biol. Phys., 2002, 7, 5–11 CrossRef CAS PubMed.
- S. R. Renn and T. C. Lubensky, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 2132–2147 CrossRef.
- H. T. Nguyen, M. Ismaili, N. Isaert and M. F. Achard, J. Mater. Chem., 2004, 14, 1560–1566 RSC.
- (a) R. A. Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907–961 RSC; (b) H. Takezoe and Y. Takanishi, Jpn. J. Appl. Phys., 2006, 45, 597–625 CrossRef CAS; (c) A. Eremin and A. Jakli, Soft Matter, 2013, 9, 615 RSC.
- L. E. Hough, M. Spannuth, M. Nakata, D. A. Coleman, C. D. Jones, G. Dantlgraber, C. Tschierske, J. Watanabe, E. Körblova, D. M. Walba, J. E. Maclennan, M. A. Glaser and N. A. Clark, Science, 2009, 325, 452 CrossRef CAS PubMed.
- H. Takezoe, Top. Curr. Chem., 2012, 318, 303–330 CrossRef CAS.
- V. P. Panov, R. Balachandran, J. K. Vij, M. G. Tamba, A. Kohlmeier and G. H. Mehl, Appl. Phys. Lett., 2012, 101, 234106 CrossRef PubMed.
- C. Dressel, T. Reppe, M. Prehm, M. Brautzsch and C. Tschierske, Nat. Chem., 2014, 6, 971–977 CrossRef CAS PubMed.
- (a) H.-C. Jeong, S. Aya, S. Kang, F. Araoka, K. Ishikawa and H. Takezoe, Liq. Cryst., 2013, 40, 951–958 CrossRef CAS; (b) H. Ocak, B. Bilgin-Eran, M. Prehm, S. Schymura, J. P. F. Lagerwall and C. Tschierske, Soft Matter, 2011, 7, 8266–8280 RSC.
- (a) L. Kovalenko, M. W. Schröder, R. A. Reddy, S. Diele, G. Pelzl and W. Weissflog, Liq. Cryst., 2005, 32, 857–865 CrossRef CAS; (b) C. Keith, A. Lehmann, U. Baumeister, M. Prehm and C. Tschierske, Soft Matter, 2010, 6, 1704–1721 RSC.
- I. Dierking and S. T. Lagerwall, Liq. Cryst., 1999, 26, 83–95 CrossRef CAS.
- C. V. Yelamaggad, A. S. Achalkumar, N. L. Bonde and A. K. Prajapati, Chem. Mater., 2006, 18, 1076–1078 CrossRef CAS.
- M. Alaasar, M. Prehm, M. Nagaraj, J. K. Vij and C. Tschierske, Adv. Mater., 2013, 25, 2186–2191 CrossRef CAS PubMed.
- P. Archer and I. Dierking, Liq. Cryst., 2006, 33, 257–265 CrossRef CAS.
- V. Novotna, M. Glogarova, V. Kozmik, J. Svoboda, V. Hamplova, M. Kaspar and D. Pociecha, Soft Matter, 2013, 9, 647–653 RSC.
- (a) S. K. Lee, L. Shi, M. Tokita and J. Watanabe, J. Phys. Chem. B, 2008, 112, 6762–6766 CrossRef CAS PubMed; (b) D. Chen, H. Wang, M. Li, M. A. Glaser, J. E. Maclennana and N. A. Clark, Soft Matter, 2014, 10, 9105–9109 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of compounds, analytical data, DSC traces, additional XRD patterns, textures and other data. See DOI: 10.1039/c5cc01592h |
| ‡ The helical twisting power is an empirical measure of the effect of chirality on LC superstructures. It depends on molecular structural parameters, conditions (e.g. T), enantiomeric purity and the type of superstructure. It is inversely proportional to the helical pitch and depends on the helicity of the molecular conformations, the energy barriers between enantiomorphic conformers and the degree of coupling of the stereogenic unit with the molecular conformational helicity.5 |
| § The birefringence in the homeotropic SmC* phases is much lower than in the SmC phase of rac-12 (see Fig. 4), indicating the presence of a helix along the layer normal being larger than the wavelength of light. |
| ¶ That no switching peak could be observed even in the SmC* phases could be due to the known weak effect of the (S)-2-methylbutyl group on polar order, leading to very small values of spontaneous polarization.5,14b Also optically, no switching can be observed; this confirms that there is no switching or it takes place by rotating around the molecular long axis. |
| || This kind of chiral SmC phase was previously reported for 4-cyanoresorcinol based BC molecules with azobenzene wings, and a local SmCsPF structure was assumed to be responsible for chirality.18 However the recent observation of chiral segregation in isotropic liquids13 supports the possibility of spontaneous chiral segregation in SmC phases with a dense packing of the aromatic cores. This chiral segregation provides a helical distortion of the layers, thus giving rise to layer distortion and leading to amplification of small chirality effects. |
| ** TGB-like twist states and TGB phases were observed for mixtures of chiral rod-like molecules with achiral bent-core molecules19 or hockey-stick compounds20 and for chiral dimesogens with odd spacers.17 A TGB-like structure has also been discussed as a possible organization in the dark conglomerate (DC) phases of achiral BC molecules,21a but only recently a DC-like random grain boundary phase of achiral hockey-stick LC was reported.21b |
| This journal is © The Royal Society of Chemistry 2015 |