Themed issue on nanoscale biomaterials
Jordan J.
Green
a and
Jason A.
Burdick
b
aDepartments of Biomedical Engineering, Ophthalmology, Neurosurgery, and Materials Science and Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, USA. E-mail: green@jhu.edu
bDepartment of Bioengineering, University of Pennsylvania, Philadelphia, PA, USA. E-mail: burdick2@seas.upenn.edu
Introduction
The development of new biomaterials is an area of research that is rapidly advancing on many fronts. This field exists at the interface between the traditional disciplines of materials science, biology, and engineering, and also encompasses emerging fields such as nanoscience and nanoengineering. Nanoscale biomaterials comprise materials designed to interact at the molecular level with biological systems. This size scale has important implications for interactions with cells and tissues, from single interactions of cellular receptors with extracellular matrix molecules to the control of cell trafficking throughout the body. With this size scale in mind, nanoscale biomaterials are being developed for a range of applications from drug delivery1 to tissue engineering.2 This themed issue focuses on diverse materials ranging from nanoparticles/nanogels to nanofibers and nanoscale coatings to molecular probes for imaging. Topics of interest include new material development, processing techniques for the fabrication of nanoscale materials, characterization of the interactions of cells with these materials, and in vivo analysis of injected nanoscale therapeutics.A unifying theme in nanoscale biomaterials research is the rational design of materials to interact with biological systems in a precise way. These biomaterials are often multi-component and multi-functional with unique material structures and chemical groups that provide specialized functions. For example, Fig. 1 (reprinted from Singh et al., a Feature Article in this issue) demonstrates a multifunctional nanoscale drug delivery system. The system begins with a nanoparticle core that could have a specialized function, such as being MRI active, or could be a template that consists of biodegradable materials that could subsequently be degraded away. To this nanoparticle core, layers of biomaterials are added in a layer-by-layer fashion. Depending on the properties of the biomaterials in these layers, internal layers can encapsulate both hydrophilic and hydrophobic drugs, biologics such as nucleic acids and proteins, imaging agents, and radiolabels. On the outside of the nanoscale drug delivery device, biomaterials such as polyethylene glycol (PEG) can provide shielding to prevent undesirable non-specific interactions. In addition, biomolecules can be conjugated to the outside biomaterial surface to impart a specialized binding function (through ligands) or environmentally triggered release (through enzymatically degradable linkages). In this manner, sophisticated nanoscale drug delivery systems can be created from constituent biomaterials.
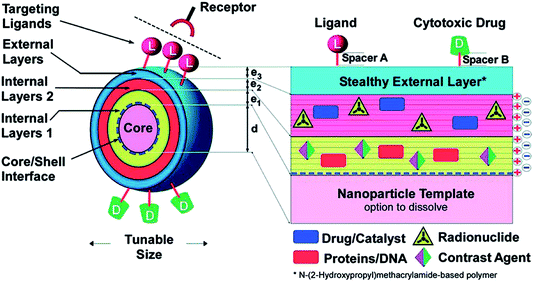 | ||
| Fig. 1 Example of a nanoparticle design that incorporates features such as ligands for targeting of particles to specific cells and tissues, drugs/protein/DNA as therapeutic cargo, and contrast agents for imaging nanoparticles in vivo. This image is reproduced from the review of Singh et al. published in this themed issue, which was reprinted from Schneider et al., 2009 (ref. 3) with permission from the ACS. | ||
The scope of materials used for nanoscale biomaterials includes metals and inorganic materials, natural biological materials, and synthetic biomaterials. Each material type has its own unique properties, such as gold nanoparticles, which can be used for imaging or photothermal therapy, and polymers, which can be made biodegradable and easily functionalized. Nanofibers and gels can contain nanoscale cues to better interact with cells, including modulating their adhesion, proliferation, and differentiation. These nanoscale biomaterials often include responsive triggers (e.g., enzymes, temperature, light, pH, magnetic field, and other modalities) that can direct their performance in vivo.
As additional information is learned from DNA sequencing efforts, disease treatments can be made increasingly stratified and more safe and effective. Nanoscale biomaterials are uniquely positioned to address challenges with conventional medicines due to their design flexibility. For example, combination nanoparticle-based treatments can deliver multiple factors (siRNA, miRNA, protein, small molecules) to targets simultaneously, to overcome multi-drug resistance and enable stem cell tracking and programming.
This themed issue provides a range of reviews and original research articles from experts in the field of nanoscale biomaterials. The topics are diverse, but unified through the design of materials at the nanoscale to impart specific functionality in biomedical applications.
Nanomaterial reviews
The themed issue begins with four important reviews on topics related to nanoscale materials. First, Dr Pfeifer and colleagues (DOI: 10.1039/c4tb01058b) provide an overview of the application of biomaterials as vaccines that are both safe and effective, towards fighting infectious diseases. Biomaterials are now being designed to elicit specific immune responses towards genetic vaccines, particularly with respect to nanomaterials.Towards the delivery of drugs for therapeutics, Dr Amiji and colleagues (DOI: 10.1039/c4tb01083c) present a review on developments in the design and application of nanoscale delivery systems for cancer therapy. Combinatorial approaches are being pursued to identify drug delivery systems that are both effective and safe. These approaches have been successful in pre-clinical studies and are moving towards clinical application. In this same area, Dr Eckmann and colleagues (DOI: 10.1039/c4tb01141d) provide a review on nanogels and their tailored properties related to targeting and therapeutic release. Nanogels have potential in numerous applications and particularly towards vascular drug targeting, where design optimization is being used to improve therapeutic performance.
With a focus on a very different application, Dr Gramlich and colleagues (DOI: 10.1039/c4tb00961d) outline a coatings approach to limit biofilm production on implants. They describe techniques that use nanoscale self-assembly of amphiphilic copolymers to disrupt bacterial adhesion and biofilm formation. This approach relies on the engineering of polymers with respect to chemistry, which influences the resulting assembly structure and performance.
Nanomaterials for gene therapy
One of the areas where nanomaterials have been extensively studied over the past decade is for non-viral gene therapy, where polymers and nanomaterials can be designed as polyplexes for trafficking of DNA into cells (e.g., to produce a therapeutic protein). There are a number of broad examples provided where nanomaterials are impacting this field. The Guelcher laboratory (DOI: 10.1039/c4tb00352g) developed a library of synthetic polymers to improve the colloidal stability of plasmid DNA polyplexes. The polymers within the library stabilized the polyplexes through hydrophobic interactions and limited colloid formation, leading to improved transfection when compared to polyethyleneimine controls. The materials were also incorporated into macroporous scaffolds, further exploiting their therapeutic potential for tissue engineering applications.Building further into non-viral gene therapy approaches, Woodworth and colleagues (DOI: 10.1039/c4tb00559g) developed triblock polymers (PEG, poly-L-histidine, poly-L-lysine) for complexing with DNA and trafficking into glioblastoma cells. The nanoparticles were effective at protecting the DNA and were able to silence tumor-specific transgene in vivo. Next, the Cheng laboratory (DOI: 10.1039/c4tb00750f) used a unique approach to incorporate a small molecule that inhibits the rho-associated protein kinase pathway to enhance the non-viral delivery of genes into human embryonic stem cells. This approach limits colony spreading to improve cell exposure to the vectors and was successful over a range of commercial transfection reagents and without affecting stem cell pluripotency. Further, the Mao laboratory (DOI: 10.1039/c4tb00967c) investigated the role that nanoparticle shape plays on non-viral transfection, using the incorporation of PEG grafts that transition a nanoparticle from worm- and rod-like structures to more condensed particles with PEG cleavage. This approach is able to de-couple the role that shape plays in nanoparticle trafficking from material charge towards transfection.
In another example of engineering nanomaterials, micelles were developed by the Tirrell laboratory (DOI: 10.1039/c4tb00977k) based on polyelectrolytes that protect nucleic acid cargo from degradation and that encompass targeting via peptide modification. The micelles were used to deliver inhibitors against dys-regulated microRNAs that promote atherosclerosis and incorporated peptide targets to fibrin within lesions or to VCAM-1. The micelle functionality was illustrated in macrophages and endothelial cells and shows great potential for treatment of atherosclerosis. The Urello lab (DOI: 10.1039/c4tb01435a) used a different approach that focused on interactions with a natural material, collagen. Specifically, they used collagen-mimetic peptide modified DNA polyplexes to control the release kinetics from collagen structures for over a month. The polyplexes remained active and transfection was enhanced in cells that were able to degrade the collagen environment, likely releasing the therapeutic as the cells migrated.
In addition to nanoparticulate formulations for gene therapy, the coating of materials with nano-scale materials may influence how cells interact with biomaterials. The Murphy laboratory (DOI: 10.1039/c4tb00957f) present an approach where 3-dimensional scaffolds are coated with minerals to alter the transfection of multipotent stem cells. This technique is performed in a screening platform to assess a range of scaffold properties with outcomes of transfection and bone morphogenetic protein-2 production by cells. The work represents a significant advance where traditional screening approaches are performed on 2-dimensional substrates and can now be performed in macroporous scaffolds.
Nanoparticles for drug delivery
Beyond their use in the delivery of DNA, nanomaterials are being exploited for the widespread delivery of a range of other therapeutics. Dr Ying and colleagues (DOI: 10.1039/c4tb00948g) designed core–shell polymers for the sustained delivery of bupivacaine for post-surgical pain relief. The particles released therapeutics at clinically useful levels for at least 2 weeks and showed no toxic outcomes or structural damage after injection and for up to 4 weeks. The MacKay laboratory (DOI: 10.1039/c4tb00979g) used an approach to encapsulate the mitogenic protein lacritin into elastin-like polypeptide based nanoparticles for delivery to the corneal epithelium. The nanoparticles influenced corneal epithelial cells in vitro and were potent when applied to an in vivo model of diabetic mice. The results were most effective when a thermally-mediated assembly was used for nanoparticle formation and lacritin encapsulation, indicating the importance of material design in therapeutic outcomes.Towards a bioresponsive approach to molecule delivery, the Zhang laboratory (DOI: 10.1039/c4tb01110d) designed liposomes that are stabilized by adsorbed chitosan-modified gold nanoparticles and are sensitive to bacterium-secreted phospholipase A2 enzyme. In the presence of the enzyme, the liposomes released their payloads in a triggered fashion, which could be abrogated in the presence of an enzyme inhibitor. The engineered liposomes could be used in numerous applications where responsive delivery features are needed. In contrast to the direct encapsulation of therapeutics, cells can be encapsulated that subsequently release therapeutic agents with time. Dr Stabler and colleagues (DOI: 10.1039/c4tb01241k) engineered alginate microcapsules with nanoscale coatings to encapsulate cells and to control permeability with coating design. More specifically, alginate microbeads were coated layer-by-layer with hyperbranched alginate and charged dendrimers to form stable coatings where the properties could be altered by criteria such as the number of coatings or chemical structures.
Nanoparticle imaging agents
In addition to the delivery of therapeutics with nano-scale materials, nanoparticles can also be used for imaging applications due to their numerous advantages in targeting and cell-binding. The Suggs laboratory (DOI: 10.1039/c4tb00975d) used gold nanoparticles that labeled either stem cells or macrophages to monitor both cell types in vivo after stem cell delivery, overcoming issues related to traditional macrophage endocytosis of nanoparticles. They used photoacoustic imaging to visualize the preferential labeling of stem cells and macrophages both in vitro and in vivo through a rat hind limb ischemia model. This technology will help to better understand differential cell populations within in vivo environments. Dr Cheng and colleagues (DOI: 10.1039/c4tb00980k) investigated cell membrane-derived nanoparticles with respect to structure–pharmacokinetic relationships, where membrane orientation was monitored using ssDNA probes. The study illustrates how molecular probes can be used to elucidate structural properties of cell membrane-derived nanoparticles and to compare different fabrication approaches.Magnetic resonance imaging (MRI) is one modality that can be used widely for imaging various disease pathologies and therapeutic interventions. Cormode and colleagues (DOI: 10.1039/c4tb01159g) developed bismuth–iron oxide nanohybrid contrast agents that can be used for both MRI and computed tomography and incorporate a dextran coating to improve biocompatibility. The contrast agents were non-toxic to cells in vitro, were biodegradable, and were effective for X-ray attenuation and as contrast agents for T2-weighted MRI. Combining the benefits of nanoscale materials for both imaging and therapeutic delivery – termed theranostics, Dr Lin and colleagues (DOI: 10.1039/c4tb00751d) developed coordination polymers that assemble with metal ions and organic ligands that could be loaded with bisphosphonates and used for MRI. The particles were also targeted to cancer cells using anisamide and their functionality was investigated in vitro.
Nanofibrous materials
Beyond nanoparticle structure, nanofibrous materials are also applicable to widespread biomedical therapies. Nanofibers present a size-scale structure that is similar in dimension to the native extracellular matrix and a high surface area that can be useful for cell interactions or molecule delivery. The Wooley laboratory (DOI: 10.1039/c4tb00909f) developed hydrogel systems with fibrillar structures that transition into sol in a reversible manner through sonication/enzymes (gel-to-sol) and heating (sol-to-gel). This was achieved through a multi-responsive triblock hydrogelator that forms β-sheet tertiary ordering.Electrospinning has been widely used to fabricate nanofibrous scaffolds from polymer solutions. The first report in this issue using this technique is from the Burdick laboratory (DOI: 10.1039/c4tb00724g) and involves the ordering of fibrous layers through the use of guest–host complexes in electrospun nanofibers and polymers that are used to adhere nanofibers together. This approach allowed the adhesion of nanofibrous mats for assembly into ordered macroscale structures and can be used to mimic the layered orientation of tissues such as myocardium and annulus fibrosus. Dr Alsberg and colleagues (DOI: 10.1039/c4tb01487a) improved electrospinning techniques through high humidity environments and postprocessing ultra-sonication to increase the porosity of nanofibrous alginate scaffolds. This enhanced porosity led to improved infiltration of fibroblasts into the center of scaffolds, towards their use as scaffolds for biomedical applications.
Summary
These reviews and manuscripts highlight important advances in the development of nanoscale materials for biological applications, ranging from nanoparticle drug delivery and imaging systems to nanostructured coatings to nanofibrous scaffolds for tissue engineering. In all of the examples, the nanoscale dimensions were important in the material design and led to improved trafficking of particles or biomimetic structures that impart specific functionality to cells. As the field develops and our understanding of these materials improve, their utility towards clinical application will develop even further.References
- N. Bertrand, J. Wu, X. Xu, N. Kamaly and O. C. Farokhzad, Adv. Drug Delivery Rev., 2014, 66, 2–25 CrossRef CAS PubMed.
- P. M. Mendes, Chem. Soc. Rev., 2013, 42, 9207–9218 RSC.
- G. F. Schneider, V. Subr, K. Ulbrich and G. Decher, Nano Lett., 2009, 9, 636–642 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


