Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[GaF3(BzMe2-tacn)] – a neutral ‘metalloligand’ towards alkali metal and ammonium cations in water†
Rajiv
Bhalla
a,
William
Levason
b,
Sajinder K.
Luthra
c,
Graeme
McRobbie
c,
Gillian
Reid
*b,
George
Sanderson
b and
Wenjian
Zhang
b
aCentre for Advanced Imaging, University of Queensland, Brisbane, Queensland 4072, Australia
bSchool of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: G.Reid@soton.ac.uk; Tel: +44-(0)23-8059-3609
cGE Healthcare UK, White Lion Road, Amersham, HP7 9LL, UK
First published on 4th September 2014
Abstract
The neutral complex, [GaF3(L)] (L = 1-benzyl-4,7-dimethyl-1,4,7-triazacyclononane, BzMe2-tacn), acts as a ‘metalloligand’ to Na+, K+ and [NH4]+ cations in aqueous solution, forming supramolecular assemblies containing significant Na/K–F and H3N+H⋯F coordination. κ1-[BF4]− and κ2-[PF6]− coordination is also evident to Na+ and K+, respectively.
Metal fluoride complexes often display quite different properties and reactivities compared to the corresponding chlorides and bromides, due to the small size and high electronegativity of the hard fluoride ligand.1 As part of our work investigating the potential of metal coordination complexes towards new classes of PET imaging agents we have reported the radio-fluorination of group 13 trichloride complexes based upon the triaza-macrocyclic ligand scaffold,2 showing that 18F incorporation into [GaCl3(L)] (L = 1-benzyl-4,7-dimethyl-1,4,7-triazacyclononane, BzMe2-tacn) occurs readily in aqueous MeCN at room temperature via halide exchange. The formation of the [Ga18/19F3(L)] is driven by the high Ga–F bond energy, and the trifluoride complex is extremely stable both in water and in phosphate buffered saline. Crystallographic studies showed that [MF3(L)] and [MF3(Me3-tacn)] are heavily hydrated and form extended supramolecular networks through strong F⋯H–OH hydrogen bonding. Earlier work by Wieghardt and co-workers had reported that first observation of the S6-symmetric methanol hexamer, crystallised within the hydrophobic cavity present in the complex [GaF3(L′)] (L′ = 1,4,7-tris(2-amino-3,5-di-tert-butylbenzyl)-1,4,7-triazacyclononane).3 Further, we found from ESI+ mass spectrometry studies on the HPLC purified radio-product [Ga18/19F3(L)] that [GaF3(L) + NH4]+ was the major ionic species observed, and proposed2 that association of [NH4]+ (from the NH4OAc(aq) HPLC mobile phase) with the highly electronegative region created by the three facial fluorides of [GaF3(L)]. A very recent report of [GdIII3MIII2] based molecular magnets derived from Gd(NO3)3·5H2O with [MIIIF3(Me3-tacn)]·xH2O (M = Cr, Fe, Ga)4 further fuelled our interest in exploiting the wider scope of the [GaF3(L)] complex to act as a robust ‘metalloligand’ towards other cations, building novel complexes with (functional) supramolecular architectures, adding a new type of building block for this type of application.5 Binary p-block fluorides such as XeF2 and AsF3 can coordinate to Ln3+ and alkaline earth dications in extreme solvents, i.e. anhydrous HF or liquid AsF3.6
In order to investigate this behaviour further, we used ESI+ MS to probe the speciation from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios of preformed [GaF3(L)] with various alkali metal cations (via the salts LiBF4, NaBF4, KPF6 and Cs2CO3) in 5
1 molar ratios of preformed [GaF3(L)] with various alkali metal cations (via the salts LiBF4, NaBF4, KPF6 and Cs2CO3) in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN
1 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. In each case peaks due to [GaF3(L) + M]+, with the correct isotopic distribution, were observed, as well as [{GaF3(L)}2 + M]+ in some cases. Similarly, addition of NH4PF6 gave [GaF3(L) + NH4]+ – see ESI.† The high affinity of the alkali metal cations for water is well-known,7 and, with the exceptions of the ubiquitous crown ether and cryptand derivatives, and group 1 cation–π(arene) complexes8 which have attracted interest due to their relevance in biological systems (such as potassium-selective channels9 and sodium-dependent allosteric regulation of serine proteases10), few coordination complexes of the group 1 cations formed in aqueous solution with neutral ligands are known.7
H2O. In each case peaks due to [GaF3(L) + M]+, with the correct isotopic distribution, were observed, as well as [{GaF3(L)}2 + M]+ in some cases. Similarly, addition of NH4PF6 gave [GaF3(L) + NH4]+ – see ESI.† The high affinity of the alkali metal cations for water is well-known,7 and, with the exceptions of the ubiquitous crown ether and cryptand derivatives, and group 1 cation–π(arene) complexes8 which have attracted interest due to their relevance in biological systems (such as potassium-selective channels9 and sodium-dependent allosteric regulation of serine proteases10), few coordination complexes of the group 1 cations formed in aqueous solution with neutral ligands are known.7
We were able to prepare directly and isolate [{GaF3(L)}2Na2(BF4)2] (1) and [{GaF3(L)}2K2(OH2)4(PF6)2]·H2O (2) by combining equimolar solutions of [GaF3(L)] and NaBF4 or KPF6, respectively, in water and allowing the products to form as colourless crystals over several days. A few (poorly diffracting) crystals of the mixed Na+/NH4+ compound [{GaF3(L)}Na(NH4)(PF6)2] (3) were also obtained from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of [GaF3(L)] and NH4PF6 in water; the adventitious Na+ most likely originating from the glassware; further evidence, however, for the high affinity of the gallium fluoride complex for the group 1 cations.
1 molar ratio of [GaF3(L)] and NH4PF6 in water; the adventitious Na+ most likely originating from the glassware; further evidence, however, for the high affinity of the gallium fluoride complex for the group 1 cations.
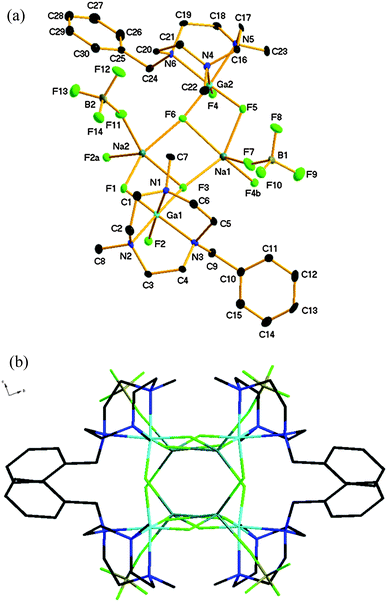
Compound 1 crystallises in the monoclinic space group P21/c with two GaF3(L) moieties and two NaBF4 units in the asymmetric unit. The structure shows (Fig. 1) two [GaF3(L)] moieties bridged by two five-coordinate, distorted square based pyramidal (τ = 0.21 (Na1), 0.23 (Na2)) sodium cations. Each sodium ion is coordinated through two μ2-bridging fluoride ligands from one [GaF3(L)] unit (κ2), one fluoride from a (κ1) BF4− ion, and a single μ3-bridging fluoride from each of two further distinct (symmetry-related) gallium moieties. This leads to an extended 1-D zig-zag chain structure. The μ3-F atoms form a Na2F2 rhombus at the core.
The Na–F bond distances involving the GaF3 unit slightly longer for the μ3-F atoms (F3 and F6) than for the μ2-F atoms. The latter are little different from those observed in [GaF3(Me3-tacn)]·4H2O,2 where the F atoms are involved in significant H-bonding with the H2O solvate. The Na–F distances lie in the ranges 2.210(3)–2.426(3) Å (Na1) and 2.200(3)–2.421(3) Å (Na2). These mostly lie within the sum of the ionic radii for Na+ and F− (1.16 and 1.19 Å respectively)11 derived from crystalline NaF. Despite the hydrophilicity of the Na+ cations, no water is retained in the crystal structure of 1.
IR spectroscopy and ESI+ mass spectrometry of compound 1 supported the formulation observed crystallographically, although in D2O solution the 1H, 19F{1H}, 23Na and 71Ga NMR resonances are not significantly different from those of the constituents in water. This indicates that 1 is extensively dissociated in water, typical of very labile alkali metal complexes.
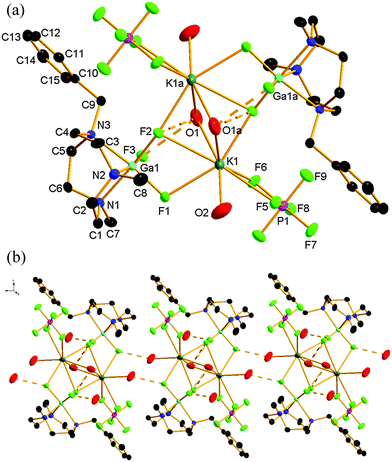
Compound 2 crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one half of a centrosymmetric tetranuclear entity (2) in the asymmetric unit. The structure also confirms coordination of the K+ to [GaF3(L)] through the fluorides (Fig. 2a). The structure is based upon eight-coordinate K+, coordinated to two F atoms from one GaF3(L) moiety (one of which is μ2, and the other μ3), one μ3-F from the second GaF3(BzMe2-tacn) unit, one terminal and two bridging OH2 ligands. The coordination environment at each K+ ion is completed by a κ2-coordinated PF6− ion. This tetranuclear (Ga2K2) species shows H-bonding interactions between the bridging OH2 ligands and two fluorides ligand of the gallium species (O1⋯F2 2.893(3), O1⋯F3 2.762(3) Å). In addition, further H-bonding is evident between the terminal OH2 ligands and a fluoride ligand from an adjacent ‘Ga2K2’ unit (O2⋯F1 2.740(4) Å), resulting in a 1D chain polymer motif (Fig. 2b). This results in a strongly H-bonded supramolecular assembly. The solvent water molecule also forms a Ga–F⋯H–OH hydrogen bond (O3⋯F3 2.729(4) Å). The K–F distances lie in the range 2.578(3) to 2.928(3) Å, comparable with the sum of the ionic radii of K+ (1.65 Å for eight coordination) and F− (1.19 Å).11
with one half of a centrosymmetric tetranuclear entity (2) in the asymmetric unit. The structure also confirms coordination of the K+ to [GaF3(L)] through the fluorides (Fig. 2a). The structure is based upon eight-coordinate K+, coordinated to two F atoms from one GaF3(L) moiety (one of which is μ2, and the other μ3), one μ3-F from the second GaF3(BzMe2-tacn) unit, one terminal and two bridging OH2 ligands. The coordination environment at each K+ ion is completed by a κ2-coordinated PF6− ion. This tetranuclear (Ga2K2) species shows H-bonding interactions between the bridging OH2 ligands and two fluorides ligand of the gallium species (O1⋯F2 2.893(3), O1⋯F3 2.762(3) Å). In addition, further H-bonding is evident between the terminal OH2 ligands and a fluoride ligand from an adjacent ‘Ga2K2’ unit (O2⋯F1 2.740(4) Å), resulting in a 1D chain polymer motif (Fig. 2b). This results in a strongly H-bonded supramolecular assembly. The solvent water molecule also forms a Ga–F⋯H–OH hydrogen bond (O3⋯F3 2.729(4) Å). The K–F distances lie in the range 2.578(3) to 2.928(3) Å, comparable with the sum of the ionic radii of K+ (1.65 Å for eight coordination) and F− (1.19 Å).11
As expected, the Ga–N bond lengths are not significantly affected by the alkali metal cation coordination in compounds 1 and 2.
Microanalytical, IR spectroscopic and ESI+ MS data from an isolated sample of 2 are consistent with the formula identified crystallographically. The IR spectra of 1 and 2 show significant broadening and splitting of the ν(BF4−) and ν(PF6−) stretching vibrations compared to the parent tetrahedral and octahedral anions, respectively, probably resulting from their coordination to the alkali metal ions.
Although the crystal data quality for compound 3 was much inferior compared to 1 and 2, analysis of the structure confirms the composition and reveals the key features of the coordination environment. The structure shows (Fig. 3) 3 is a chain polymer with two alternating types of six coordinate Na+ ions, both with F6 coordination; one type involving two κ2-GaF3(L) units and two κ1-[PF6]− anions, the second involving two κ1-GaF3(L) units and two κ2-[PF6]− anions. Interestingly, the [NH4]+ cations also form significant F⋯H–N hydrogen bonding interactions with adjacent Fs both from GaF3(L) and from [PF6]− anions. The presence of both Na+ and [NH4]+ ions in 3 is also supported by ESI+ mass spectrometry data on the isolated product.
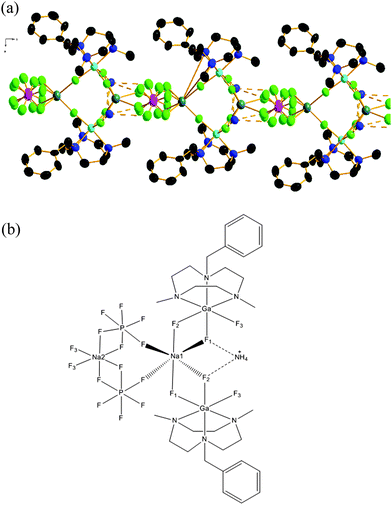 | ||
| Fig. 3 (a) View of the structure of a portion of the polymeric structure formed by 3. Ellipsoids are drawn at 50% probability level. H-atoms on the BzMe2-tacn ligand are omitted for clarity; those of the NH4+ cation were not located (see ESI†). Colour key: turquoise = Ga, teal = Na, pink = P, green = F, blue = N, black = C. (b) Line drawing illustrating the coordination environment at Na+ and the F⋯HNH3+ hydrogen bonding interactions in 3. | ||
We have shown that [GaF3(L)] can function as a very effective F-donor ‘metalloligand’ towards alkali metal cations in water, leading to highly unusual and distinct structural types. The results suggest that rational development of new multimetallic frameworks and assemblies based upon metal fluoride coordination complexes as metalloligands towards other inorganic and organic cations should be possible.
This work was funded by EPSRC and GE Healthcare through a CASE studentship (G.S.). The authors thank Dr M. E. Light for help with the crystallographic analyses.
Notes and references
- S. L. Benjamin, W. Levason and G. Reid, Chem. Soc. Rev., 2013, 42, 1460 RSC; H. C. S. Clark and J. H. Holloway, in Advanced Inorganic Fluorides, ed. T. Nakajima, B. Žemva and A. Tressaud, Elsevier, Oxford, 2000, ch. 3 CrossRef CAS; N. M. Doherty and N. W. Hoffman, Chem. Rev., 1991, 91, 553 CrossRef CAS; E. F. Murphy, R. Murugavel and H. W. Roesky, Chem. Rev., 1997, 97, 3425 CrossRef PubMed.
- R. Bhalla, C. Darby, W. Levason, S. K. Luthra, G. McRobbie, G. Reid, G. Sanderson and W. Zhang, Chem. Sci., 2014, 5, 381 RSC.
- F. N. Penkert, T. Weyhermüller and K. Wieghardt, Chem. Commun., 1998, 557 CAS.
- K. S. Pedersen, G. Lorusso, J. J. Morales, T. Weyhermüller, S. Piligkos, S. K. Singh, D. Larsen, M. Schau-Magnussen, G. Rajaraman, M. Evangelisti and J. Bendix, Angew. Chem., Int. Ed., 2014, 53, 2394 CrossRef CAS PubMed.
- G. Kumar and R. Gupta, Chem. Soc. Rev., 2013, 42, 9403 RSC.
- M. Tramšek and B. Žemva, J. Fluorine Chem., 2006, 127, 1275 CrossRef PubMed and references therein.
- T. P. Hanusa, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2004, ch. 1, vol. 3 Search PubMed.
- See for example: B. Werner, T. Kräuter and B. Neumüller, Organometallics, 1996, 15, 3746 CrossRef CAS; G. B. Deacon, T. C. Feng, P. C. Junk, B. W. Skelton and A. H. White, J. Chem. Soc., Dalton Trans., 1997, 1181 RSC.
- R. L. Nakamura, J. A. Anderson and R. F. Gaber, J. Biol. Chem., 1997, 272, 1011 CrossRef CAS PubMed.
- Q. D. Dang, E. R. Guinto and E. Di Cera, Nat. Biotechnol., 1997, 15, 146 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
Footnote |
† Electronic supplementary information (ESI) available: Full experimental details for the compounds reported, in situ ESI+ MS data from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios of [GaF3(L)] with alkali metal salts and NH4PF6, plus all the crystallographic and spectroscopic data for all products. CCDC 1008581–1008583. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc05603e 1 molar ratios of [GaF3(L)] with alkali metal salts and NH4PF6, plus all the crystallographic and spectroscopic data for all products. CCDC 1008581–1008583. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc05603e |
| This journal is © The Royal Society of Chemistry 2014 |