The rechargeable aluminum-ion battery
N.
Jayaprakash
,
S. K.
Das
and
L. A.
Archer
*
School of Chemical and Biomolecular Engineering, Cornell University, Ithaca, New York 14853-5201
First published on 3rd November 2011
Abstract
We report a novel aluminium-ion rechargeable battery comprised of an electrolyte containing AlCl3 in the ionic liquid, 1-ethyl-3-methylimidazolium chloride, and a V2O5 nano-wire cathode against an aluminium metal anode. The battery delivered a discharge capacity of 305 mAh g−1 in the first cycle and 273 mAh g−1 after 20 cycles, with very stable electrochemical behaviour.
Since the early 1990s, lithium ion batteries based on a carbonaceous material such as graphite as the anode, a lithiated metal oxide (LiMO, e.g.LiCoO2) cathode, and an aprotic liquid electrolyte have been the subjects of intense scientific and commercial interest for portable electronics applications. In recent years, the demand for secondary/rechargeable batteries with higher operating voltages, improved cycling stability, higher power densities, enhanced safety, and lower initial and life cycle costs has increased to meet new needs for smaller, lighter, more powerful electronic devices. Growing interest in hybrid electric vehicles (HEV) and plug-in hybrid electric vehicles (PHEVs) designed to simultaneously reduce dependence on fossil fuel-derived energy and to lower the carbon footprint of humans compounds the current need for advanced, cost-effective electrical energy storage technologies.1 Due to their high energy density and low self-discharge rate, Li-ion batteries are considered the most promising technology for meeting these demands for the foreseeable future.2–4
Aluminium is the most abundant metal and the third most abundant element in the earth's crust. An aluminium-based redox couple, which involves three electron transfers during the electrochemical charge/discharge reactions, provides competitive storage capacity relative to the single-electron Li-ion battery.5–7 Additionally, because of its lower reactivity and easier handling, such an aluminium-ion (Al-ion) battery might offer significant cost savings and safety improvements over the Li-ion battery platform. Aluminium has consequently long attracted attention as an anode in the Al–air battery because of its high theoretical Ampere-hour capacity and overall specific energy.8–11 Although these values are reduced in a practical battery because of the inability to operate aluminium and the air cathode at their thermodynamic potentials, and because water is consumed in the discharge reaction, the practical energy density still exceeds that of most commercially available rechargeable battery systems.12 The inherent hydrogen generation of the aluminium anode in aqueous electrolytes introduces additional limitations, which have been met by designing the batteries as reserve systems with the electrolyte added just before use, or as mechanically rechargeable batteries with the aluminium anode replaced after each discharge. Even with these adjustments, reliable electrically rechargeable aluminium/air batteries are considered unfeasible using aqueous electrolytes, due to the high corrosion and hydrogen evolution of aluminium in the electrolyte, which leads to a sharp reduction of the anodic efficiency. Furthermore, several studies have shown that in aqueous media Al/air batteries an oxide film covers the surface of the aluminium anode, which decreases the anodic voltage and cell efficiency.13 The literature is replete with studies investigating the suitability of ionic liquid-based electrolytes for electrodeposition of aluminium.14 A battery technology exploiting an Al-ion conducting ionic liquid electrolyte has been recently reported to show some promise.15
Herein, we report on a novel Al-ion battery system using V2O5 nano-wires as the cathode against an aluminium metal anode in an ionic liquid (IL), 1-ethyl-3-methylimidazolium chloride, -based electrolyte.16 To the best of our knowledge, the Al-ion battery configuration reported in this communication is the first to offer evidence of stable electrochemical behaviour with extended cycle life. We attribute these features to the synergistic effect of a suitable electrolyte, V2O5 nano-wire cathode and Al anode. Specifically, a key requirement for achieving high energy density of an Al-ion battery is an electrolyte having good ionic conductivity for Al3+, a wide electrochemical stability window in the presence of metallic aluminium, and which wets and permeates the pores of the metal oxide cathode. The apposite electrolyte should also exhibit reversible electrochemical deposition and dissolution of aluminium.
Based on the aforementioned studies of electrodeposition of aluminium in room temperature ILs,14AlCl3 dissolved in 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) was used as the electrolyte in the current study to examine the operation of the Al-ion battery at room temperature (25 °C). This electrolyte possesses different degrees of Lewis acidity depending on the [EMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 ratio, which provides an additional degree of freedom in tuning its properties. During discharge the prevalent AlCl4− anion in the electrolyte will react with the Al anode to form Al2Cl7− complex species, which reacts with the cathode to form an aluminium intercalated V2O5 discharge product. An acidic electrolyte composition with 1.1
AlCl3 ratio, which provides an additional degree of freedom in tuning its properties. During discharge the prevalent AlCl4− anion in the electrolyte will react with the Al anode to form Al2Cl7− complex species, which reacts with the cathode to form an aluminium intercalated V2O5 discharge product. An acidic electrolyte composition with 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of AlCl3 to ([EMIm]Cl) was found to yield effective electrochemical deposition and dissolution of aluminium and was therefore used for the study. To verify the role played by the AlCl3–[EMIm]Cl electrolyte, electrochemical investigation of the same battery system was also performed with an electrolyte containing aluminium trifluromethanesulfonate (Al triflate) dissolved in a conventional aprotic liquid mixture PC/THF (1
1 molar ratio of AlCl3 to ([EMIm]Cl) was found to yield effective electrochemical deposition and dissolution of aluminium and was therefore used for the study. To verify the role played by the AlCl3–[EMIm]Cl electrolyte, electrochemical investigation of the same battery system was also performed with an electrolyte containing aluminium trifluromethanesulfonate (Al triflate) dissolved in a conventional aprotic liquid mixture PC/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). In contrast to the AlCl3–[EMIm]Cl electrolyte system, no electrochemical activity was observed in the measured voltage range −0.75–4.2 V, underscoring the importance of the IL-based electrolyte.
1 v/v). In contrast to the AlCl3–[EMIm]Cl electrolyte system, no electrochemical activity was observed in the measured voltage range −0.75–4.2 V, underscoring the importance of the IL-based electrolyte.
The V2O5 nanowires used for the cathode were prepared by a hydrothermal method.17 In a typical synthesis, 0.364 g of commercial V2O5 powder (Sigma-Aldrich) and 30 ml of DI H2O were mixed under vigorous magnetic stirring at room temperature, and then 5 ml 30% H2O2 (Sigma-Aldrich) was added to this mixed solution and kept continuously stirred for 30 min. Finally a transparent orange solution was obtained. The resultant solution was then transferred to a 40 ml glass lined stainless steel autoclave and heated at 205 °C for 4 days. The product was washed with anhydrous ethanol and distilled water several times. Finally, it was dried at 100 °C for 12 h and then annealed at 500 °C for 4 h in air. The synthesized product was characterized by Transmission Electron Microscopy (TEM, Tecnai, T12, 120 kV), powder X-ray diffraction (Scintage X-ray diffractometer with Cu Kα radiation), cyclic voltammetry (Solartron's Cell Test model potentiostat under the scan rate of 0.2 mV s−1), and galvanostatic electrochemical charge–discharge analysis (Maccor cycle life tester, under the potential window 2.5–0.02 V).
The V2O5 cathode slurry was made by mixing 85% of the synthesized V2O5 nano-wires, 7.5% super-p carbon and 7.5% of PVDF binder in NMP dispersant. Electrodes were produced by coating the slurry onto a 10 micron stainless steel current collector at 105 °C for 1 h initially and at 100 °C for 4 h in a vacuum oven. Since the acidic electrolyte used has the tendency to etch copper, stainless steel (301 type stainless steel, McMaster Carr, USA) was used as the current collector. The resulting slurry-coated stainless steel foil was roll-pressed and the electrode was reduced to the required dimensions with a punching machine. Preliminary cell tests were conducted on 2032 coin-type cells, which were fabricated in an argon-filled glove box (AlCl3 is highly reactive), using 10 micron Al metal as the counter electrode and a Whatman glass microfiber separator. The electrolyte solution was 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 anhydrous AlCl3 in 1-ethyl-3-methylimidazolium chloride.
1 anhydrous AlCl3 in 1-ethyl-3-methylimidazolium chloride.
The phase purity and degree of structural order of the synthesized V2O5 was studied using a powder X-ray diffraction (XRD) pattern shown in Fig. 1a. The XRD obtained is in good agreement with the standard JCPDS pattern (File No. 89-0612) and shows the existence of phase pure orthorhombic V2O5 with Pmmn space group. The absence of any undesirable peaks demonstrates the presence of a phase pure product and the miller indices (hkl) of all the characteristic peaks are marked as per the standard pattern. Fig. 1b and c shows the transmission electron microscopy (TEM) image of the as synthesized V2O5 nano-wires. It is apparent that the synthesis procedure yields uniform and nearly monodispersed nanostructures having uniform diameters throughout their entire length and sharp, well-defined surfaces.
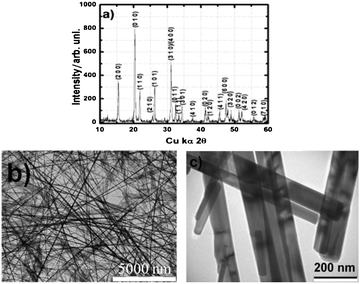 | ||
| Fig. 1 (a) XRD pattern and (b, c) TEM images of the V2O5 nano-wires used for the Al-ion secondary battery cathode. | ||
To evaluate the feasibility of the electrolyte and the synthesized V2O5 nano-wires for Al-ion battery applications, electrochemical properties were examined by cyclic voltammetry and galvanostatic cycling analysis. Fig. 2a and b show the cyclic voltammograms of the V2O5 cathode against the Al metal anode in two different electrolytes: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v of Al triflate in PC/THF (Fig. 2a) and 1.1
1 v/v of Al triflate in PC/THF (Fig. 2a) and 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of AlCl3 in ([EMIm]Cl) (Fig. 2b) at room temperature. As mentioned earlier, no electrochemical activity was observed for the Al-ion battery using Al triflate in PC/THF as the electrolyte and V2O5 nano-wire cathode in the measured voltage range of −0.75–4.2 V. On the other hand, a pair of cathodic and anodic peaks were observed for the Al-ion battery with V2O5 nano-wire cathode and AlCl3 in the [EMIm]Cl electrolyte under the potential window of 2.5–0.02 V. The CV pattern shown in Fig. 2b exhibited a cathodic peak at ∼0.45 V and a corresponding anodic peak at ∼0.95 V, respectively, which may be attributed to the insertion/deinsertion of Al3+ ions into and from the orthorhombic crystal lattice structure of V2O5 nano-wires. Virtually no change in the peak position or peak current value was observed in the cyclic voltammogram shown in Fig. 2b even after 20 scans, which substantiates the electrochemical stability of the battery. For this reason, AlCl3 in [EMIm]Cl was chosen as the electrolyte for discharge/charge studies.
1 molar ratio of AlCl3 in ([EMIm]Cl) (Fig. 2b) at room temperature. As mentioned earlier, no electrochemical activity was observed for the Al-ion battery using Al triflate in PC/THF as the electrolyte and V2O5 nano-wire cathode in the measured voltage range of −0.75–4.2 V. On the other hand, a pair of cathodic and anodic peaks were observed for the Al-ion battery with V2O5 nano-wire cathode and AlCl3 in the [EMIm]Cl electrolyte under the potential window of 2.5–0.02 V. The CV pattern shown in Fig. 2b exhibited a cathodic peak at ∼0.45 V and a corresponding anodic peak at ∼0.95 V, respectively, which may be attributed to the insertion/deinsertion of Al3+ ions into and from the orthorhombic crystal lattice structure of V2O5 nano-wires. Virtually no change in the peak position or peak current value was observed in the cyclic voltammogram shown in Fig. 2b even after 20 scans, which substantiates the electrochemical stability of the battery. For this reason, AlCl3 in [EMIm]Cl was chosen as the electrolyte for discharge/charge studies.
![Typical cyclic voltammograms of Al-ion battery using V2O5 nano-wire cathode and aluminium anode in (a) 1 : 1 v/v of Al triflate in PC/THF and (b) 1.1 : 1 molar ratio of AlCl3 in ([EMIm]Cl) at a sweep rate of 0.2 mV s−1.](/image/article/2011/CC/c1cc15779e/c1cc15779e-f2.gif) | ||
Fig. 2 Typical cyclic voltammograms of Al-ion battery using V2O5 nano-wire cathode and aluminium anode in (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v of Al triflate in PC/THF and (b) 1.1 1 v/v of Al triflate in PC/THF and (b) 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of AlCl3 in ([EMIm]Cl) at a sweep rate of 0.2 mV s−1. 1 molar ratio of AlCl3 in ([EMIm]Cl) at a sweep rate of 0.2 mV s−1. | ||
To further evaluate the electrochemical properties of the designed Al-ion battery, a galvanostatic discharge/charge reaction was performed at the cell voltage of 2.5–0.02 V at a constant current drain of 125 mA g−1. The open circuit voltage of the Al-ion battery was found to be 1.8 V. Fig. 3a displays the voltage vs. time plot of the Al-ion battery, wherein no change in the potential of the Al3+ insertion/extraction plateau was observed. Fig. 3b shows the voltage vs. capacity plot of the Al-ion battery which demonstrates a well defined and very stable Al3+ insertion plateau at ∼0.55 V. In the first cycle, the battery exhibited an Al3+ ion insertion capacity of 305 mAh g−1 against 273 mAh g−1 at the end of 20 cycles. These values are somewhat lower than the theoretical capacity of V2O5 against the Al3+ ion, which is estimated to be 442 mAh g−1 considering a simple three electron transfer reaction (Al + V2O5 ↔ AlV2O5). Fig. 3c shows the galvanostatic cycling performance of the Al-ion battery, which nicely shows a high degree of reversibility and good capacity retention. The coulombic efficiency of the cell is not as high as would be needed for long-term practical use in a secondary battery, but its stability is quite encouraging. Significant crystallography and voltammetry studies are underway to understand intercalation of aluminium into V2O5 and other layered oxides. These studies will help shed light on the effect of variables such as the cell current density and electrolyte formulation on the coulombic efficiency and practical specific energy achievable in the Al-ion battery. Indeed based on the specific capacities observed experimentally, one might conclude that only about 0.7 moles of Al3+ ions participate in the actual redox reaction. As in the case of Li-ion secondary batteries, we anticipate significant opportunities for nanoscale engineering and chemical design of the Al-ion battery cathode to increase the overall cell potential. Additionally, we anticipate as significant efforts to pioneer ionic liquid and other Al-conducting electrolytes to enhance cell performance at high voltages and high current drains.
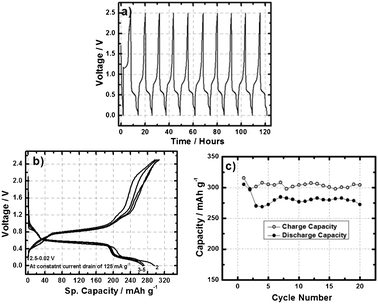 | ||
| Fig. 3 (a) Voltage vs. time (b) voltage vs. sp. capacity and (c) cycle life plot of Al-ion battery containing aluminium anode, V2O5 nano-wire cathode in ionic liquid under the potential window 2.5–0.02 V and at a constant current drain of 125 mA g−1. | ||
In conclusion, we report the first example of a functional Al-ion rechargeable battery. The battery uses V2O5 as the cathode against an aluminium metal anode in an ionic liquid-based electrolyte. When evaluated in galvanostatic cycling and cyclic voltammetry measurements, the battery displayed promising electrochemical features with stable cycling behaviour over 20 charge–discharge cycles. The theoretical energy density of the Al-ion battery with V2O5 as the cathode against aluminium metal as the anode was determined to be 240 Wh kg−1, which is not enough to power very much. But considering the other attractive attributes of an aluminium based secondary battery platform, the energy density of this first Al-ion cell is enough to initiate a global search for new materials and cell designs which we anticipate will lead to rapid and sustained improvements.
This publication was based on work supported in part by the Energy Materials Center at Cornell, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Basic Energy Sciences, under Award Number DE-SC0001086, and by Award No. KUS-C1-018-02, made by King Abdullah University of Science and Technology (KAUST). Facilities available through the Cornell Center for Materials Research (CCMR) were also used for this study.
Notes and references
- G. M. Whitesides and G. W. Crabtree, Science, 2007, 315, 796 CrossRef CAS.
- Y. K. Sun, S. T. Myung, B. C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320 CrossRef CAS.
- T. Zhang, N. Imanishi, Y. Shimonishi, A. Hirano, Y. Takeda, O. Yamamoto and N. Sammes, Chem. Commun., 2010, 46, 1661 RSC.
- T. Ohzuku, M. Nagayama, K. Tsuji and K. Ariyoshi, J. Mater. Chem., 2011, 21, 10179 RSC.
- Q. Li and N. J. Bjerrum, J. Power Sources, 2002, 110, 1 CrossRef CAS.
- X. Y. Ren, Battery Bimonthly, 1997, 27, 85 CAS.
- J. Singer and W. L. Fielder, Am. Met. Mark., 2000, 108, 4 Search PubMed.
- M. L. Doche, F. N. Cattin, R. Durand and J. J. Rameau, J. Power Sources, 1997, 65, 197 CrossRef CAS.
- X. Y. Ren, Battery Bimonthly, 1997, 27, 85 CAS.
- R. S. M. Patnaik, S. Ganesh, G. Ashok, M. Ganesan and V. Kapali, J. Power Sources, 1994, 50, 331 CrossRef CAS.
- E. J. Rudd and D. W. Gibbons, J. Power Sources, 1994, 47, 329 CrossRef CAS.
- D. Linden and T. B. Reddy, Handbook of Batteries, 3rd edn, 1995, McGraw-Hill Search PubMed.
- Y. Tang, L. Lu, H. W. Roesky, L. Wang and B. Huang, J. Power Sources, 2004, 138, 313 CrossRef CAS.
- T. Jiang, M. J. Chollier Brym, G. Dubé, A. Lasia and G. M. Brisard, Surf. Coat. Technol., 2006, 201, 1 CrossRef CAS.
- M. P. Paranthaman, G. M. Brown, X. Sun, J. Nanda, A. Manthiram and A. Manivannan, Abstract #314, 218th ECS Meeting.
- S.K. Das and L.A. Archer, The Rechargeable Aluminum-ion battery, Cornell University Invention Disclosure, Docket #5506.
- T. Zhai, H. Liu, H. Li, X. Fang, M. Liao, L. Li, H. Zhou, Y. Koide, Y. Bando and D. Golberg, Adv. Mater., 2010, 22, 2547 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
