Influence of humic acid and UV-irradiation on iron-based nanoparticle toxicity in Girardia tigrina†
Natasha
Yadav
ab,
Anurag
Nath
ab,
Pushplata Prasad
Singh
ab,
Himadri B.
Bohidar
a,
Damien L.
Callahan
c,
Antoine M.
Dujon
bd,
Luis O. B.
Afonso
b and
Aaron G.
Schultz
 *b
*b
aNational Centre of Excellence for Advanced Research in Agricultural Nanotechnology, TERI-Deakin Nanobiotechnology Centre, Sustainable Agriculture Division, The Energy and Resources Institute (TERI), DS Block, India Habitat Centre, Lodhi Road, New Delhi, 110003, India
bSchool of Life and Environmental Sciences, Deakin University, Geelong, Victoria 3217, Australia. E-mail: aaron.schultz@deakin.edu.au
cSchool of Life and Environmental Sciences, Deakin University, Burwood, Victoria 3125, Australia
dCREEC/(CREES), MIVEGEC, Unité Mixte de Recherches, IRD 224-CNRS 5290-Université de Montpellier, Montpellier, France
First published on 11th October 2024
Abstract
The rapid advancement of nanotechnology has led to the increasing application of metal oxide nanoparticles (NPs) in various fields, including agriculture, where they offer potential benefits such as improved nutrient delivery and pest control. However, concerns about their environmental impact necessitate a comprehensive assessment of their safety. This study investigated the potential toxic effects of iron-based nanoparticles (NPs) on freshwater planarian and the influence of abiotic factors such as humic acid (HA) and UV exposure on their toxicity. Three different types of iron-based NPs were tested, including commercially available Sigma iron oxide magnetic NPs (Sig_IOMNPs), biologically synthesized BS_IOMNPs and Zn–Fe and bulk FeSO4. Sigma and biogenic nanoparticles had predominantly magnetite (Fe3O4) structure whereas Zn–Fe possessed a bimetallic conformation. Interaction of these NPs with abiotic factors (HA and UV light) led to an increase in their hydrodynamic diameter. In contrast to the commercial sources (Sig_IOMNPs and bulk FeSO4), the biologically synthesized NPs did not cause any acute or sublethal toxicity to the planarian when alone or in combination with HA and UV. These results suggest that biologically synthesized iron-based NPs (Zn–Fe and BS_IOMNPs) may be a safe alternative to conventional bulk iron-based fertilizers. This study highlights the importance of investigating the physicochemical changes of NPs in environmentally realistic conditions and assessing their potential toxicity to aquatic organisms. These findings can contribute to the development of safe and sustainable agricultural practices, promoting the use of iron-based NPs as a new generation of fertilizers.
Environmental significanceIron-based nanomaterials (NMs) can be useful as new fertilizers to increase agricultural yields, with biological synthesis emerging as a safer method of their production. However, there remains a critical knowledge gap regarding the environmental behavior and risks posed by these agriculturally relevant NMs. Our study addresses this gap by investigating the complex interactions between iron-based nanoparticles, humic acid, and UV irradiation, elucidating their toxicity to freshwater planaria as a model organism. Our findings reveal no acute toxicity from biologically synthesized iron-based NPs, offering crucial insights into their environmental fate and potential risks in aquatic ecosystems. Our research not only advances the fundamental understanding of nanoparticle behavior but also promotes a sustainable approach to nanotechnology in environmental science, facilitating the safe development of nanomaterial products for agriculture. |
1. Introduction
The application of nanomaterial (NMs) is expanding in diverse disciplines, including agriculture, environment, and industry because of the fast advancement of biocompatible nanotechnology.1 Metal oxide nanoparticles (NPs) are particularly attractive for agricultural use due to their high surface reactivity, small size, and large surface area, which enhance nutrient delivery, pest control, and soil interactions. These properties allow for more efficient, controlled, and environmentally friendly agrochemical applications.2 Metal oxide NPs have been studied extensively to determine their long-term relevance for plant development and agricultural productivity.3,4 Some studies have shown that metal oxide NPs, including zinc oxide, copper oxide and titanium oxide NPs, can reduce fertilizers' costs by increasing crop productivity.5 Among various metal oxide NPs, iron NPs have gained attention in agriculture due to their propensity for improving plant development.3 It is possible these NPs could replace conventional iron fertilizers, which have a plethora of drawbacks including high cost, and the tendency to leach out or runoff into soil and ultimately decreasing their efficiency.6–10 The effects of iron oxide NPs on plant development and productivity have been the subject of many recent studies11–15 and have been demonstrated to promote plant growth and development.9 On the other hand, little is known about the potential negative impact of iron-oxide NPs in the environment.NMs have been classified as emerging contaminants by the European Union and the United States Environmental Protection Agency. They have also been listed as potentially hazardous to the environment by the Nano missions of India, because of their high reactivity.16 It is possible that increased production and use of these iron-based NPs might result in their release into aquatic environments through surface runoff from the soil. The main sink of NPs discharged from agricultural areas are aquatic systems.17 NPs may undergo transformations when applied to agricultural fields and before reaching aquatic ecosystems. Once reaching such ecosystems, NPs may undergo additional transformations, including aggregation, dissolution, sedimentation, sulfidation and redox reaction due to their interaction with abiotic [light, ions, natural organic matter (NOM), temperature, and pH] and biotic factors present in terrestrial and aquatic environments,18–20 which may affect their physicochemical properties and impact their availability and toxicity.21,22 For example, it has been reported that environmentally transformed zinc oxide NPs exhibit increased toxicity to mammalian cells as a result of the release of Zn2+(aq) ions.23 Similar results have been documented for Ag NPs as well.24 To establish the ecotoxicity of iron oxide NPs, several aquatic life forms at different levels of the food web have been studied. According to these studies,25–30 deleterious effects have been found in all aquatic species (algae, daphnia, mussel, and carp). Baumann et al. (2014) and García et al. (2011) found that iron-oxide NPs induced acute toxicity in Daphnia magna, which significantly reduced survival rates.25,26 Similarly, Lei et al. (2016), Magro et al. (2018), Remya et al. (2015), and Taze et al. (2016) documented toxicity in a range of aquatic species, including green algae, Labeo rohita (carp), and Mytilus galloprovincialis (mussel).27–30 This toxicity led to growth inhibition, decreased photosynthetic efficiency in algae, survival decline, developmental issues in Daphnia, alterations in hematological parameters, disrupted ion-regulation, impaired gill Na+/K+ ATPase activity in Labeo rohita (Carp), and oxidative stress, resulting in cellular damage in Mytilus galloprovincialis. During these studies, it was determined that NP exposure resulted in increased formation of reactive oxidative species (ROS), as well as DNA damage in organisms, subsequently resulting in their toxicity.31 Based on these previous studies, iron-based NPs may pose a significant risk to aquatic biota, and it is, therefore, important to assess the toxicity risks of new types of iron-based NPs before they are commercialised or released into the environment.
Recently, studies on boron nitride NPs32 and Ag NPs,33 have revealed the importance of freshwater planarian as a model for evaluating nanotoxicity. Planarians have mainly been used as a model for stem cell over the past 20 years.34–36 Additionally, they have ecological significance due to their dual position in the food webs as predator and prey. They are also simple to work with and maintain in the laboratory.37 Furthermore, planarians are epibenthic species, interacting with the surface layer of sediments due to their ciliary movement,38 making them an ideal model for assessing the toxicity of NPs on freshwater aquatic species.39 It has been shown that exposure of planarians to iron oxide (Fe3O4) NPs did not affect their stem cells population and regenerative capacity.40 Nevertheless, this study solely examined the effects of chemically synthesized iron oxide NPs without considering any abiotic factors. As far as we know, no study has investigated the effects of chemically or biologically synthesised iron-based on planarian, either in pristine laboratory conditions or in the presence of environmental abiotic factors, including sunlight (UV radiations) or the presence of natural organic matter (NOM).
Sunlight or UV exposure is also an important abiotic factor. Surface oxidation processes, ROS production, and dissolving rates of metal-based NPs can all be impacted by exposure to UV or light.41 UV-treated ZnO nanoparticles were phototoxic to Artemia salina, leading to mortality and Zn2+ accumulation.42 Another study demonstrated that the toxicity of TiO2 NPs in zebrafish (Danio rerio) was influenced by light conditions, highlighting the role of UV radiation in modifying NPs toxicity in aquatic environments.43 Solar UV radiation increased ZnO nanoparticle toxicity to Daphnia magna through ROS generation and particle dissolution, with CaCl2 and N-acetylcysteine mitigating toxicity to varying degrees under UV irradiation.44 All these findings highlight the importance of considering environmental UV radiation in assessing NPs toxicity and risk in aquatic ecosystems. Furthermore, NOM (e.g., humic acid) are ubiquitous in surface water and are well known to alter surface characteristics of NPs, which in turn affects their transformation processes in the environment.45–47 The toxicity and bioavailability of NP are consequently further impacted by their interaction with NOM in aquatic media.48–50 Recent studies evaluating the interactions of NPs with NOM have been conflicting results, with some showing increased effects of toxicity of NPs to aquatic organisms in the presence of NOM,51–53 while others reporting reduced toxicity (protective effects48–50) or no effects on toxicity.54,55 Collectively, these studies suggest that the role of NOM in NPs toxicity is complex and needs to be further investigated.48–50
The toxicity of NPs in freshwater planarian has not yet been studied in relation to the effects of either HA or UV. The toxicity risks of iron-based NPs important to agriculture were studied in this research using a freshwater planarian as a model, along with the individual and combined effects of abiotic components, HA, and UV radiation. This study examined both acute (survival) and sublethal toxicity of the NPs, including effects on animal morphology (head loss (acephalia), crenelated margins, an oval body, and shortening of auricles) and animal behaviour to identify the risks of biologically produced iron-based NPs on freshwater environments.
2. Materials and methods
2.1 Materials and characterization of NPs in test media
The commercial iron oxide nanoparticles (Fe3O4: spherical shape and less than 100 nm) used in this study were purchased from Sigma Aldrich, USA, (product number 637106). The iron sulphate heptahydrate NP (FeSO4·7H2O) was purchased from HiMedia, India (product number GRM1377). The silver (Ag) nanospheres coated with Econix PVP and measuring 5 nm in size were purchased from Nanocomposix, USA. The Suwannee River humic acid standard, a product of the International Humic Substances Society (IHSS), USA, (product number 3S101H). Planarian growth media (PGM) used in the study was natural spring water purchased from Coles, Australia. Nitric acid (HNO3) was purchased from Sigma Aldrich, USA, (product number 22571; 99.99%). There were no trace elements or metal contaminants present. The deionized water used was sourced from MilliQ system with the conductivity of 18.2 MΩ. All procedures were performed at room temperature (20 °C) unless stated otherwise.In this study, three types of iron-based NPs were used. Biologically synthesized iron-oxide magnetic nanoparticle (BS_IOMNPs) and nano zinc–iron nanocomposite (nano Zn–Fe) were synthesized in-house using previously published biological synthesis methods.56,57 Sigma iron-oxide magnetic nanoparticles (Sig_IOMNPs) were purchased from a commercial supplier (Sigma Aldrich, USA). NPs were characterized earlier using various analytical techniques, including scanning and transmission electron microscopy (SEM and TEM), dynamic light scattering (DLS), Fourier Transformed Infra-Red (FTIR) spectroscopy, X-Ray Diffraction (XRD), and inductively coupled plasma mass spectrometry (ICP/MS).56 Table S1 (ESI†) provides the summary properties of all these NPs. The hydrodynamic size, zeta potential, and polydispersity index of these NPs were determined using a Zetasizer ZS90 (Malvern, UK; backscatter mode) at a concentration of 200 μg mL−1 in PGM. Additionally, all test NPs were also characterised in the presence of 20 μg mL−1 of HA and under exposure of 8 h of UV irradiation.
2.2 Freshwater planarian maintenance and culture
Wild freshwater planarian, Girardia tigrina of size 8–10 mm, were collected from St. Augustine's lagoon (Highton, Victoria, Australia, 38°11′30′′S, 144°18′48′′N) in Waurn Ponds, Victoria (Australia), in November 2023 and were stored in 500 mL non-hazardous food grade plastic containers containing 250 mL of spring water (Coles natural spring water) at 20 °C at a density of approximatively 1 individual per 5 mL.37 Except while feeding, cultures were kept in close to total darkness. Beef liver was fed as a dietary source twice a week. The beef liver was minced and kept in aliquots at 4 °C until required. PGM and containers were replaced twice weekly, once after feeding and again 2 to 3 d afterwards. Planarians were deprived of food for 4–6 d before the experiments to establish a constant physiologic condition. The animals were maintained as per the protocol previously described.332.3 Experimental procedures and exposure conditions
The experimental design involved the placement of four randomly chosen planarian into each well of a 6-well plate which was filled with PGM (5 mL in each well). Two independent experimental series were conducted, with untreated planarian serving as the control in each case. In the treatment group, PGM was removed from the wells and replaced with 5 mL of exposure solutions. The first experiment involved exposure of planarians to increasing concentrations (0, 25, 50, 100, 250 and 500 μg mL−1) of BS_IOMNPS, nano Zn–Fe, Sig_IOMNPs, or bulk FeSO4 at 20 °C in an incubator for 96 h. For the positive control, planarians were exposed to 25 μg mL−1 concentration of silver nanoparticles (Ag NPs). Silver nanoparticles (Ag NPs) were used as a positive control in the experiments to observe sublethal toxicity effects on planarians. These effects were anticipated based on previous studies by a member of our laboratory, which demonstrated the toxicological impact of Ag NPs on planarians. Specifically, within 48 hours of exposure to Ag NPs at a dosage of 12.5 μg mL−1, significant morphological changes such as head loss, shortening of auricles, and an oval body were observed.58–60 A stereomicroscope was used to assess their survival at 6, 24, 30, 48, 54, 72, 78 and 96 h. The experiment was performed in triplicates. Therefore, the overall survival % following exposure to NP was evaluated using 12 planarians. Sub-lethal toxicity in planarian is marked by many morphological alterations, including acephalia (head loss), an oval body with crenelated margins, and shortening of the auricles under stress.61 A stereomicroscope was used to assess these morphological alternations at the stipulated time intervals mentioned earlier.In the second set of experiment, the effects of abiotic factors on NP toxicity in planarian were investigated. HA (at a concentration of 20 μg mL−1), UV, or a combination of both were introduced in addition to the test NPs at concentrations of 50 and 200 μg mL−1. An HA only control (20 μg mL−1) was also included. To assess the effects of sunlight on NP toxicity, planarians were exposed to UV light for 12 h during the day and 12 h without UV during the night at 20 °C and 80% humidity levels. The UV irradiance intensities were 125 W cm−2 and 60 W cm−2 for UV-B and UV-A respectively, based on previous study by Priyam et al. (2022) that optimized sunlight on a sunny day for studying the effect of UV irradiance on NP toxicity in zebrafish embryos.62
In order to further investigate potential behavioural changes, the mobility of planarian was also evaluated after exposure to the NPs using light exposure to stimulate movement by following a previously published method.63 The 6-well plates containing the 4 planarians in each well exposed to increasing doses of the NPs were placed gently on graph paper with 1 mm square grids under a 12 megapixels HD camera and planarian movement was recorded for 5 min directly after a light source was turned on. Videos were later analysed and the number of times planarian crossed or recrossed a square during a five minute period was used to determine individual mobility. The average total distance that each planarian travelled per minute was used to quantify their locomotory activity. This movement analysis was performed at 24 and 72 h after exposure to the NPs.
2.4 Quantification of NP uptake by planarian
All planarians from the dose–response NP toxicity experiments were collected after 96 h and were stored at −80 °C to quantify NP uptake. These planarians were first acid digested to prepare the sample for single particle inductively coupled plasma mass spectrometry (spICP-MS) analysis. The digestion was performed using a modified protocol described by Žemberyová et al.64 The planarians were transferred to a 1.5 ml tube and twice washed with DI water to eliminate any residual NP attached to the surface of the planarian. Further, 1.5 mL of 70% nitric acid was then added to the weighed sample. Digestion was performed in a heat block utilising at 95 °C for 2–3 h. Following digestion, the material was suspended in 1% nitric acid and subjected to spICP-MS analysis (NexION 350X, PerkinElmer, USA).2.5 Statistical analysis
GraphPad Prism was used for all statistical analysis (v9.5). A two-way ANOVA and Dunnett's multiple comparison test were used to determine if the results on morphological alternation, and behavioural abnormalities were statistically different from the untreated control. The two independent variables (factors) in this analysis were ‘treatment type’ and ‘time point’. Morphological alterations and behavioural abnormalities served as the dependent variables. The survival rates of both control and experimental groups at 96 h post-treatment was also evaluated using pairwise Chi-square tests with a Bonferroni correction to account for multiple comparisons. p value ≤0.05 was considered significant for each case.3. Results
3.1 Effect of test media on NPs characteristics
The variations in hydrodynamic diameter and zeta potential of NPs distributed in various test medium conditions (DI water, PGM, presence of HA and UV separately or jointly) is presented in Fig. 1. Regardless of the test medium conditions, the hydrodynamic diameter of all tested NPs increased significantly at 96 h (p < 0.05) when compared to time zero (within media). In DI water, the nano Zn–Fe diameter increased from 1366 ± 254 nm at 0 h to 2976 ± 134 nm at 96 h and in PGM the nano Zn–Fe increased from 1974 ± 130 nm at 0 h to 3293 ± 472 nm at 96 h (Fig. 1a). Similarly, time-dependent increases in hydrodynamic diameter were observed for each of the other NPs (Fig. 1a, c and e). Moreover, when comparing the test media conditions, all NPs exhibited larger hydrodynamic diameters when dispersed in PGM compared to DI water. For BS_IOMNPs (24 and 96 h) and Sig_IOMNPs, the increase was shown to be statistically significant at 0, 24, and 96 h (Fig. 1c and e).Each test NP had a distinct hydrodynamic diameter when dispersed in PGM, in the presence of HA and UV together or separately. At 96 h in PGM + UV exposure, nano Zn–Fe and BS_IOMNPs had hydrodynamic diameters of 2936 ± 767 nm and 3199 ± 252 nm, respectively. However, in the absence of UV exposure in PGM, the hydrodynamic diameter of nano Zn–Fe and BS_IOMNPs was 3293 ± 99 nm and 4450 ± 99 nm, respectively (Fig. 1a and c). While the hydrodynamic diameter for Sig_IOMNPs (3199 ± 290 nm at 96 h) was observed to be the same to the PGM condition only (with no UV exposure) (Fig. 1e). This further shows that UV radiation has no impact on the hydrodynamic diameter of test NPs.
Interestingly, the hydrodynamic diameter of each test NP was not significantly affected by the presence of HA. However, a slight reduction in hydrodynamic diameter was seen for nano Zn–Fe (3054 ± 678 nm) and BS_IOMNPs (3317 ± 498 nm) in the presence of HA at 96 h compared to PGM only (Fig. 1a and c). For Sig_IOMNPs, compared to PGM, a slight increase in hydrodynamic diameter (3572 ± 251 nm) was seen at 96 h in the presence of HA (Fig. 1e). Similar to the HA condition only, the presence of both HA and UV together in PGM had no significant impacts on the hydrodynamic diameter of the test NPs.
The zeta potential of each NP measured under various test medium conditions, as well as in the presence of HA and UV, was consistent with the hydrodynamic diameter measurements for each NP. As the hydrodynamic diameter of each test NP increased over time, the zeta potential value increased in all test media conditions. In DI water, the zeta potential value of nano Zn–Fe shifted from −19.86 ± 0.64 mV at 0 h to −16.50 ± 1.27 mV at 96 h and in PGM, zeta potential values change from −22.56 ± 1.32 mV at 0 h to −18.53 ± 0.56 mV at 96 h. Similar significant changes in the zeta potential over time were also observed for all other NPs and each of test media conditions (Fig. 1d and f). In ESI,† Fig. S.1–S.6 report the size distribution of the NPs versus intensity, number, and volume frequency curves. The positive NP control, 5 nm Ag NPs, were also characterised at a concentration of 200 μg mL−1 in DI water and PGM to evaluate their time and media dependent behaviour. Ag NPs were also observed aggregating with a significant increase in hydrodynamic size over time in PGM, similar to iron-based NPs. However, it was shown that particles were most stable in DI water. Further, the zeta potential values of the Ag NPs became increased over time in PGM, which was in accordance with the increasing hydrodynamic size of the NPs (Fig. S.7†). Additionally, Fig. S.8 in ESI† demonstrates the size distribution of the NP versus intensity, number, and volume frequency curves observed for Ag NPs.
3.2 Effect on survival of planarian
Exposure to increasing concentrations of iron-based NPs (nano Zn–Fe, BS_IOMNPs, and Sig_IOMNPs) and bulk ferrous sulphate (FeSO4) had no impact on the survival of planarian up to 96 h (Fig. 2a–d). However, planarians exposed to the positive control (Ag NPs: 25 μg mL−1), had their survival rate significantly reduced to 58% at 96 h (with p < 0.05; Fig. 2a–d).Exposure of planarian to nano Zn–Fe and BS_IOMNPs in the presence of UV and HA (20 μg mL−1), or in the presence of both UV and HA, had no effect on survival with 100% survival rate throughout the experiment (Fig. S.9a–d†). However, survival rate was significantly decreased after 72 h exposure to 50 and 200 μg mL−1 doses of Sig_IOMNPs and bulk FeSO4 in the presence of HA and UV (Fig. S.9c and d†).
3.3 Effect on morphology of planarian
Planarian treated with Ag NPs at a dosage of 12.5 μg mL−1 were considered as a positive control to observe sublethal toxicity effects on planarian morphology, including head loss, oval body, and shortening of the auricles.3.4 Effect on planarian mobility
At the 24 and 72 h exposure time points, planarian locomotion was recorded. No statistically significant effect on planarian mobility (average distance travelled) was observed when exposed to nano Zn–Fe and BS_IOMNPs (Fig. 3a and b). However, treatment with 200 μg mL−1 Sig_IOMNPs and FeSO4 (12.5, 25, 50, 100 and 200 μg mL−1) caused a significant decreased in planarian movement within 24 h of exposure (Fig. 3c and d). Moreover, as anticipated, exposure of planarian to the Ag NPs (positive control) caused an inhibition of planarian movement (with no planarian moving after exposure to light stimulation, Fig. 3a–d).Planarian exposed to nano Zn–Fe, BS_IOMNPs and Sig_IOMNPs (50 and 200 μg mL−1) in the presence of HA, UV or both HA and UV, had no significant impact on their mobility when compared to negative controls (Fig. 3e and f). Exposure of planarian to bulk FeSO4 (50 and 200 μg mL−1) together with both HA and UV significantly reduced planarian mobility within 24 h of treatment (Fig. 3h).
3.5 Uptake and effect on elemental distribution
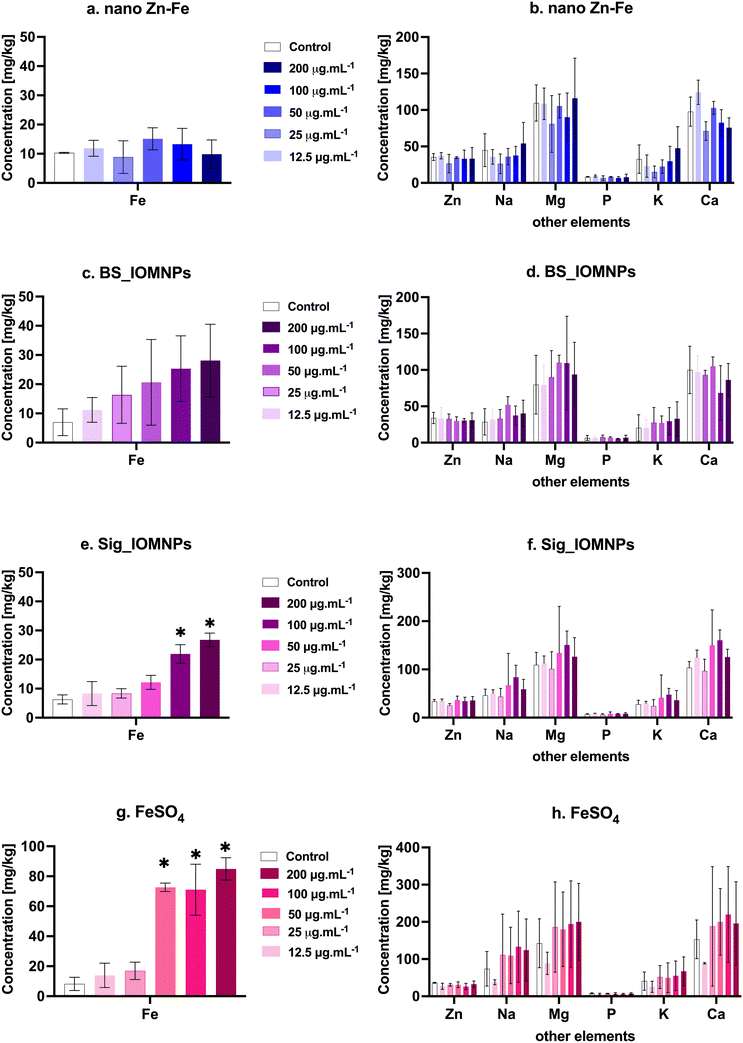
A dose-dependent significant increase in Fe concentration was observed in planarian exposed to BS_IOMNPs, Sig_IOMNPs, and FeSO4 (Fig. 4c, e and g). BS_IOMNPs exhibited enhanced uptake, rising from 11.2 mg kg−1 to 28.1 mg kg−1 as the dosage increased from 12.5 μg mL−1 to 200 μg mL−1. Likewise, the uptake of Sig_IOMNPs and FeSO4 also displayed a significant increase from 8.3 to 26.7 mg kg−1 and 11.9 to 84.9 mg kg−1 as the dosage escalated from 12.5 μg mL−1 to 200 μg mL−1, respectively. Furthermore, it was observed that the uptake of these test materials did not significantly affect the concentration of other crucial elements (Na, Mg, Ca, P, Zn, and K) in the planarian, indicating no effect on ion-regulation (Fig. 4b, d, f and h).4. Discussion
After being released into the environment NPs go through a series of transformation processes that could significantly change their size, surface charge, and reactivity, which in turn can influence their possible detrimental implications on the biota.21,41 The NPs inorganic surface interface allows them to interact with a broad range of biological systems comprised of protein, metabolites, and ions.65 These interactions can change their biological actions, that may eventually have unanticipated, detrimental, and toxic effects.66,67 When subjected to experimental media, many NPs have a propensity to agglomerate, which changes their impact on biological systems by changing their sensitivity and bio-accessibility.40 All test NPs underwent DLS characterisation in PGM, and with the presence of HA and UV exposure.All iron-based NPs were shown to have varied hydrodynamic size and zeta potential in response to distinct types of test media (DI water and PGM) and in the presence of abiotic variables HA and UV exposure used in the study. Aggregation for all test NPs in both test media conditions was observed over time. The steady increase in hydrodynamic diameter over time in DI water may be driven by the magnetic properties of iron-based NPs, which stimulates particle to particle collision, and thus encourages their aggregation, and finally leads to the formation of large aggregates.68 In contrast, the increased NP aggregation found in PGM compared to DI water may be due to the presence of the water's high ionic strength and the test NPs' magnetic properties. Comparable results from earlier investigations showed that the size of the iron oxide NPs increased over time as the ionic strength of the dispersion medium increased.69 It has also been demonstrated in the previous studies as well. For Instance, Ag NPs aggregated in test aquatic media with high ionic strength used for Daphnia magna growth.70 Further, HA was observed to slightly reduce the hydrodynamic diameter of nano Zn–Fe and BS_IOMNPs in PGM and this could be mediated by the HA surface coating on NPs, which inhibits aggregation through processes of electrostatic surface charge stabilisation.71 In a prior study, it was demonstrated that iron oxide NP aggregates disintegrated over time when exposed to high concentrations of HA (50 and 100 mg L−1).71 The increased surface charge driven by the absorption of HA served as the stimulant for the disaggregation of the NP aggregates and it was reported that the rate of disaggregation increased with HA concentration and decreased with time.72 Similar to this, the size of the test NP was seen to be reduced by HA at 24 h in the current study; however, with prolonged time, HA influence was seen to be reducing, leading into the increase in size compared to 24 h. There is no prior research on the impact of UV exposure on the size of iron-based NPs and limited studies are available on metal oxide NPs.62,73–75 The iron-based NPs were not found to be significantly affected by UV exposure, however, the nano Zn–Fe and BS_IOMNPs were shown to have smaller hydrodynamic diameters when exposed to UV. Whereas for Sig_IOMNPs no effect was observed. This is consistent with the previous study by Priyam et al. (2022), they reported no effect of UV radiation on the size of P-based NPs.62 These results further suggest that the type, size, and properties of the dispersion medium of NPs significantly affected their behaviour in the environment. Their size and bioavailability in the environment may both be impacted by interactions between particles and other abiotic components present in the water. Therefore, the interaction of NPs with abiotic factors like UV exposure and HA may be more significant in understanding how NPs affect aquatic organisms.
Thus, planarians were exposed to several different iron-based NPs at variable environmental relevant and some higher concentrations in PGM, and in the presence of abiotic factors (UV radiations and HA), in order to assess their toxicity in more environmentally realistic conditions. No discernible variations in the survival rates and morphology changes, with or without abiotic stimuli (UV radiations and HA) were identified after 5 d (96 h) of continuous exposure to nano Zn–Fe and BS_IOMNPs and the negative control (no NP treatment was applied). In addition, commercially purchased Sig_IOMNPs and bulk FeSO4 were also shown to have no effect on the survival rate and morphological alternations of the planarian up to 96 h in the absence or presence of HA or UV light independently planarian. Similarly, study by Tran et al., the only study to date in literature to investigate the detrimental effects of iron oxide NPs on planarians reported no evidence for their negative influence on the survival and behavioural changes of planarians.40 On the contrary, planarians treated with 25 μg mL−1 Ag NPs (5 nm) considered as a positive control showed a substantial decrease in their survival percentage within 24 h of treatment. Additionally, exposure to Ag NPs has also caused morphological alternations (head loss, oval body, and shortening of auricles) in the planarian within 24 h of treatment. Furthermore, exposure to Sig_IOMNPs and FeSO4, at the higher concentrations (50 and 200 μg mL−1), although higher than typical environmental levels, was used to simulate worst-case scenarios in environmental studies. In the presence of HA and UV light together, these conditions were observed to affect planarian survival at the observed time points of 72, 78, and 96 h. In our study, the hydrodynamic sizes of AgNPs aggregates were observed to be smaller than 100 nm (see Fig. S.7†), which is substantially smaller compared to the other tested NPs, whose hydrodynamic sizes were greater than 1000 nm. This size difference likely contributes to the higher toxicity observed in AgNPs, as smaller NPs are more readily taken up by biological systems. Therefore, AgNPs were selected as a positive control to provide a benchmark for high toxicity, allowing for a clearer comparison of the toxic effects of the other tested NPs.
Additionally, under the combined action of HA and UV exposure, head loss, an oval body, and shortening of the auricles were also observed to be evident at 50 and 200 μg mL−1 and time points 72, 78 and 96 h. Though, the impact of iron-based NMs on planarian in the presence of HA and UV has not been previously documented, however some studies with other organisms have revealed HA and UV light induced toxicity of metal oxide NPs such as ZnO and TiO2.50,76–78 In a study by Dasari et al. (2010), exposure to TiO2 and ZnO NPs in the presence of both HA and UV radiation inhibited the development of an assemblage of aquatic bacteria. In this study, the authors suggested that the toxicity of TiO2 NPs was associated with the release of ROS when exposed to sunlight, whereas the same impact of ZnO NPs was attributed to the release of metal ions (Zn2+) when exposed to the same parameters.76 A study conducted by Wee et al. (2020) provided additional confirmation of the increased release of Zn2+ ions under the impact of sunlight exposure and HA.79 Additionally, Bar-Ilan et al. (2013) demonstrated that zebrafish embryos exposed to lower concentrations of TiO2 NPs containing HA under simulated solar irradiation exhibited toxicity and DNA oxidative damage compared to zebrafish embryos exposed to TiO2 NPs without HA.50 All these investigations supported the idea that exposing aquatic species to metal oxide NPs in the presence of HA and sunlight can promote the formation of ROS and the release of metal ions, which in turn affects the toxicity of the NPs towards the exposed species. On the contrary, in a study by Wormington et al. (2017), NOM at a concentration of 5 mg L−1 significantly reduced the toxicity of photoreactive-TiO2 NPs to Daphnia magna when exposed to UV radiation by inhibiting the oxidation of ROS. However, it has also been shown in this same study that NOM, at high dosages of >10 mg L−1, may absorb approx. 17 to 80% of UV from the system, reducing the toxicity through UV attenuation.78 As a result, these intriguing differences in the toxicity of metal oxide NMs in the presence of ambient abiotic variables have drawn attention to the importance of NP synthesis pathways and size in determining the biological interactions of the metal oxide NPs. In line with this, biogenically produced nano Zn–Fe and BS_IOMNPs had no impact on survival or morphological abnormalities of planarians, when HA and UV were present either separately or combined, in our investigation. However, it was observed that under the combined action of HA and UV radiation, Sig_IOMNPs from chemical sources and FeSO4 caused morphological abnormalities and had significant impact on the survival of planarians. This difference in toxicity may be attributed to the distinct surface properties resulting from their synthesis methods. Biogenic NPs, synthesized using fungal cell-free extracts, tend to have biocompatible surfaces due to the formation of a bio-corona during synthesis. This bio-corona may render the NPs less toxic to organism by reducing their reactivity and interaction with cellular components.80 The lack of impact on planarian by these biogenic NPs suggests that their surfaces are less likely to induce harmful interactions with planarian. Conversely, chemically synthesized NPs, such as Sig_IOMNPs, often lack such bio-corona layers and may have surfaces more reactive due to the presence of harmful chemicals used during synthesis.
Furthermore, the potential sub-lethal effects of iron-based NPs on planarian behaviour were assessed by quantifying their mobility (total distance travelled by each planarian in the treatment well per min) in response to light. No dose-dependent impact of NPs (nano Zn–Fe, BS_IOMNPs, Sig_IOMNPs) and bulk FeSO4 was observed on the planarian mobility at both 24 and 72 h timepoints compared to the negative control (untreated). However, in the case of the positive control (treated with Ag NPs), planarian mobility was observed to be significantly impacted. Throughout the light exposure, no planarians were observed to be swimming in all of the Ag NP treatments. Additionally, Sig_IOMNPs (200 μg mL−1) and FeSO4 (12.5, 25, 50, 100 and 200 μg mL−1) were also found to significantly affect the planarian mobility at observation time point 24 h. When compared to the negative control, however, there was no discernible difference in their mobility after 72 h. According to the study previously mentioned, iron oxide NPs have been reported to have no impact on planarian's ability to move freely and escape from a light source.40 Like morphological abnormalities, Sig_IOMNPs and FeSO4 have also been shown in the study to impact the movement of planarians under the influence of abiotic conditions. Planarian mobility was measured to be affected by the combination of HA and UV, at 200 μg mL−1 of Sig_IOMNPs and 50 and 200 μg mL−1 of bulk FeSO4. There is no existing research on the impact of iron-based NPs or any other metal oxide on planarian when combined with HA and UV radiation. Recent research has found that exposure to zinc oxide nanoparticles is toxic to planarians, significantly reducing their movement. This decrease in movement is due to neurobehavioral effects, which lead to morphological changes caused by disruptions in muscle contractions controlled by the nervous system.63 Similar findings have been reported for graphene oxide NPs, where exposure to planarian caused a reduction in their mobility because of the neurotoxin activity of the particles.81 Given that both Sig_IOMNPs and bulk FeSO4 from chemical sources may have morphological abnormalities followed by decreases in mobility at concentrations of 50 and 200 μg mL−1, it is expected that these materials may also function as neurotoxins when exposed to planarian. Further, this could be complimented with the spICP-MS results, which clearly indicate the higher uptake of these materials at concentrations (50 and 200 μg mL−1), when compared to nano Zn–Fe and BS_IOMNPs.
Overall, the findings from this study suggest that biologically synthesized iron-based NPs (nano Zn–Fe and BS_IOMNPs) do not exhibit acute toxicity to freshwater planarians and show limited evidence of sub-lethal effects under the specific environmental conditions and concentrations tested. This observation suggests that Girardia tigrina may exhibit notable tolerance to metal NPs. This tolerance could be related to their adaptation to their storm water pond habitat, which might contribute to their increased resilience to metal pollutants.82 Also, planarians secrete mucus that can act as a protective barrier to contaminants, which may also explain their tolerance, especially to NPs, as they can be “trapped” in the mucus layer and not be internalised.83,84 However, since our study did not investigate regenerative abilities, further research would be necessary to explore the potential role of regeneration in their tolerance. Additionally, given that the study evaluated effects on only one species and a limited number of sub-lethal endpoints at the organismal level, further research involving multiple species and a broader range of sub-lethal endpoints is needed to comprehensively assess the potential ecological risks of these nanoparticles. However, it's important to acknowledge the limitations of this study, particularly the need to assess the protocol's applicability across other species of planarian with differing susceptibilities to metal exposure. For instance, the comparison with Cura pinguis, a local planarian species with limited regenerative capacity,85 could provide valuable insights into the broader ecological implications of metal-based NPs toxicity. Additionally, future research is also necessary to examine the processes underlying each of the sublethal impacts of chemically produced NPs, such as morphological abnormalities and reduced mobility, under environmental stress conditions. Future research should also include studies on the uptake and elimination of these NPs by planarians. Even though in this study the uptake of the NPs was measured, it is also important to obtain information on how planarians eliminate these NPs, as lower body concentrations measured in planarians can result from lower uptake but also from faster elimination.
Moreover, gaining insight into the effects of these NPs in freshwater planarian under environment conditions paves way for future investigations on higher species in the trophic levels.
5. Conclusion
In this study, the effects of pristine and transformed iron-based NPs on the acute and sub-lethal toxicity in freshwater planarian were investigated in vivo, with consideration given to abiotic factors such as HA and UV light. The hydrodynamic sizes of the NPs increased when treated with or without these factors, and their physicochemical characteristics were also observed to be altered. Exposure of planarian to pristine NPs did not result in any mortality or sublethal effects. However, Sig_IOMNPs and Bulk FeSO4 caused significant mortality and sublethal effects at certain concentrations (50 and 200 μg mL−1) under the influence of HA and UV together. The study highlights the importance of assessing NP toxicity under environmentally realistic conditions, as abiotic factors can greatly influence their behavior and toxicity in aquatic environments. The findings provide valuable information for the development of safer and more environmentally friendly iron-based NPs as fertilizers in the future.Data availability
All supporting data is available in the ESI.† All other experimental data that supports the findings of this study is available from the corresponding author upon reasonable request.Author contributions
Conceived and designed of the experiments: Aaron Schultz, Luis O. B. Afonso, and Natasha Yadav. Wet-lab work and data compilation: Natasha Yadav. Planarian sample collection and culture maintenance: Natasha Yadav and Anurag Nath. Data analyses and manuscript writing: Natasha Yadav, Aaron Schultz, and Prof. Damien L. Callahan. Critical inputs and finalization of the manuscript: Natasha Yadav, Pushplata Prasad Singh, Himadri B. Bohidar, Damien L. Callahan, Antoine M. Dujon, Luis O. B. Afonso, and Aaron G. Schultz.Conflicts of interest
The authors disclose a conflict of interest: nano Zn–Fe is the nanomaterial developed at the TERI-Deakin Nanobiotechnology Centre (TDNBC), located in Gurgaon, India. Nano Zn–Fe is the intellectual property of TDNBC.Acknowledgements
This research is supported by the Department of Biotechnology, India (grant no. BT/NNT/28/SP30280). Additionally, the research activities were supported by the TERI-Deakin Nanobiotechnology Centre in Gurugram, India, and Deakin University in Victoria, Australia.References
- V. N. Le, Y. Rui, X. Gui, X. Li, S. Liu and Y. Han, Uptake, transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton, J. Nanobiotechnol., 2014, 12(1), 1–15 CrossRef.
- A. Huang, Y. He, Y. Zhou, Y. Zhou, Y. Yang and J. Zhang, et al., A review of recent applications of porous metals and metal oxide in energy storage, sensing and catalysis, J. Mater. Sci., 2019, 54(2), 949–973 CrossRef CAS.
- D. Maity, U. Gupta and S. Saha, Biosynthesized metal oxide nanoparticles for sustainable agriculture: next-generation nanotechnology for crop production, protection and management, Nanoscale, 2022, 14(38), 13950–13989 RSC.
- S. Hyder, M. Ul-Nisa, H. Shahid, F. Gohar, A. S. Gondal and N. Riaz, et al., Recent trends and perspectives in the application of metal and metal oxide nanomaterials for sustainable agriculture, Plant Physiol. Biochem., 2023, 107960 CrossRef CAS.
- H. Singh, A. Sharma, S. K. Bhardwaj, S. K. Arya, N. Bhardwaj and M. Khatri, Recent advances in the applications of nano-agrochemicals for sustainable agricultural development, Environ. Sci.: Processes Impacts, 2021, 23(2), 213–239 RSC.
- M. Zia-ur-Rehman, A. Naeem, H. Khalid, M. Rizwan, S. Ali and M. Azhar, Responses of plants to iron oxide nanoparticles, Nanomater. Plants, Algae, Microorg., 2018, 221–238 CAS.
- G. Ch, N. Ntalli, U. Menkissoglu-Spiroudi and C. Dendrinou-Samara, Essential Metal-Based Nanoparticles (Copper/Iron NPs) as Potent Nematicidal Agents against Meloidogyne spp, J. Nanotechnol. Res., 2019, 1(2), 44–58 Search PubMed.
- H. Singh, A. Sharma, S. K. Bhardwaj, S. K. Arya, N. Bhardwaj and M. Khatri, Recent advances in the applications of nano-agrochemicals for sustainable agricultural development, Environ. Sci.: Processes Impacts, 2021, 23(2), 213–239 RSC.
- F. Fatima, A. Hashim and S. Anees, Efficacy of nanoparticles as nanofertilizer production: a review, Environ. Sci. Pollut. Res., 2021, 28, 1292–1303 CrossRef CAS PubMed.
- S. Shirsat and S. K, Iron oxide nanoparticles as iron micronutrient fertilizer—Opportunities and limitations, J. Plant Nutr. Soil Sci., 2023, 187(5), 565–588 CrossRef.
- T. M. Linh, N. C. Mai, P. T. Hoe, L. Q. Lien, N. K. Ban and L. T. T. Hien, et al., Metal-based nanoparticles enhance drought tolerance in soybean, J. Nanomater., 2020, 4056563 CAS.
- K. Jeyasubramanian, U. U. G. Thoppey, G. S. Hikku, N. Selvakumar, A. Subramania and K. Krishnamoorthy, Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles, RSC Adv., 2016, 6(19), 15451–15459 RSC.
- Y. Feng, V. D. Kreslavski, A. N. Shmarev, A. A. Ivanov, S. K. Zharmukhamedov and A. Kosobryukhov, et al., Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) Plants, Plants, 2022, 11(14), 1894 CrossRef.
- R. Noor, H. Yasmin, N. Ilyas, A. Nosheen, M. N. Hassan and S. Mumtaz, et al., Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress, Chemosphere, 2022, 292, 133201 CrossRef CAS PubMed.
- T. Raiesi-Ardali, L. Ma'mani, M. Chorom and A. Moezzi, Improved iron use efficiency in tomato using organically coated iron oxide nanoparticles as efficient bioavailable Fe sources, Chem. Biol. Technol. Agric., 2022, 9(1), 1–16 CrossRef.
- K. Varner, State of the science literature review: nano titanium dioxide environmental matters, US Environmental Protection Agency, Office of Research and Development, 2010 Search PubMed.
- H. Chen, Metal based nanoparticles in agricultural system: behavior, transport, and interaction with plants, Chem. Speciation Bioavailability, 2018, 30(1), 123–134 CrossRef CAS.
- Y. Yin, X. Yang, X. Zhou, W. Wang, S. Yu and J. Liu, et al., Water chemistry controlled aggregation and photo-transformation of silver nanoparticles in environmental waters, J. Environ. Sci., 2015, 34, 116–125 CrossRef CAS.
- X. Zou, P. Li, J. Lou, X. Fu and H. Zhang, Stability of single dispersed silver nanoparticles in natural and synthetic freshwaters: Effects of dissolved oxygen, Environ. Pollut., 2017, 230, 674–682 CrossRef CAS.
- N. Odzak, D. Kistler and L. Sigg, Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments, Environ. Pollut., 2017, 226, 1–11 CrossRef CAS PubMed.
- M. Amde, J.-f. Liu, Z.-Q. Tan and D. Bekana, Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review, Environ. Pollut., 2017, 230, 250–267 CrossRef CAS PubMed.
- P. Jadhav, N. Muhammad, P. Bhuyar, S. Krishnan, A. S. Abd Razak and A. Zularisam, et al., A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: Applications and limitations, Environ. Technol. Innovation, 2021, 23, 101526 CrossRef.
- M. M. Wang, Y. C. Wang, X. N. Wang, Y. Liu, H. Zhang and J. W. Zhang, et al., Mutagenicity of ZnO nanoparticles in mammalian cells: Role of physicochemical transformations under the aging process, Nanotoxicology, 2015, 9(8), 972–982 CrossRef CAS.
- S. Kittler, C. Greulich, J. Diendorf, M. Köller and M. Epple, Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions, Chem. Mater., 2010, 22(16), 4548–4554 CrossRef CAS.
- J. Baumann, J. Köser, D. Arndt and J. Filser, The coating makes the difference: Acute effects of iron oxide nanoparticles on Daphnia magna, Sci. Total Environ., 2014, 484, 176–184 CrossRef CAS PubMed.
- A. García, R. Espinosa, L. Delgado, E. Casals, E. González and V. Puntes, et al., Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests, Desalination, 2011, 269(1–3), 136–141 CrossRef.
- C. Lei, L. Zhang, K. Yang, L. Zhu and D. Lin, Toxicity of iron-based nanoparticles to green algae: effects of particle size, crystal phase, oxidation state and environmental aging, Environ. Pollut., 2016, 218, 505–512 CrossRef CAS PubMed.
- M. Magro, M. De Liguoro, E. Franzago, D. Baratella and F. Vianello, The surface reactivity of iron oxide nanoparticles as a potential hazard for aquatic environments: A study on Daphnia magna adults and embryos, Sci. Rep., 2018, 8(1), 1–15 CAS.
- A. S. Remya, M. Ramesh, M. Saravanan, R. K. Poopal, S. Bharathi and D. Nataraj, Iron oxide nanoparticles to an Indian major carp, Labeo rohita: Impacts on hematology, iono regulation and gill Na+/K+ ATPase activity, J. King Saud Univ., Sci., 2015, 27(2), 151–160 CrossRef.
- C. Taze, I. Panetas, S. Kalogiannis, K. Feidantsis, G. P. Gallios and G. Kastrinaki, et al., Toxicity assessment and comparison between two types of iron oxide nanoparticles in Mytilus galloprovincialis, Aquat. Toxicol., 2016, 172, 9–20 CrossRef CAS.
- P. P. Fu, Q. Xia, H. M. Hwang, P. C. Ray and H. Yu, Mechanisms of nanotoxicity: generation of reactive oxygen species, J. Food Drug Anal., 2014, 22(1), 64–75 CrossRef CAS.
- A. Salvetti, L. Rossi, P. Iacopetti, X. Li, S. Nitti and T. Pellegrino, et al., In vivo biocompatibility of boron nitride nanotubes: effects on stem cell biology and tissue regeneration in planarians, Nanomedicine, 2015, 10(12), 1911–1922 CrossRef CAS PubMed.
- L. Kustov, K. Tiras, S. Al-Abed, N. Golovina and M. Ananyan, Estimation of the toxicity of silver nanoparticles by using planarian flatworms, ATLA, Altern. Lab. Anim., 2014, 42(1), 51–58 CrossRef CAS.
- A. Zeng, H. Li, L. Guo, X. Gao, S. McKinney and Y. Wang, et al., Prospectively Isolated Tetraspanin(+) Neoblasts Are Adult Pluripotent Stem Cells Underlying Planaria Regeneration, Cell, 2018, 173(7), 1593–608 e20 CrossRef CAS.
- P. G. Barghouth, M. Thiruvalluvan, M. LeGro and N. J. Oviedo, DNA damage and tissue repair: What we can learn from planaria, Semin. Cell Dev. Biol., 2019, 89, 145–159 CrossRef PubMed.
- P. W. Reddien and A. S. Alvarado, Fundamentals of planarian regeneration, Annu. Rev. Cell Dev. Biol., 2004, 20, 725–757 CrossRef CAS.
- H. Klaassen, S. Tissot, J. Meliani, J. Boutry, A. Miltiadous and P. A. Biro, et al., Behavioural ecology meets oncology: quantifying the recovery of animal behaviour to a transient exposure to a cancer risk factor, Proc. R. Soc. B, 2016, 2024(291), 20232666 Search PubMed.
- P. Rompolas, R. S. Patel-King and S. M. King, Schmidtea mediterranea: a model system for analysis of motile cilia, Methods Cell Biol., 2009, 93, 81–98 CAS.
- T. Knakievicz, Planarians as invertebrate bioindicators in freshwater environmental quality: the biomarkers approach, Ecotoxicol. Environ. Contam., 2014, 9(1), 1–12 Search PubMed.
- T. A. Tran, M. Hesler, O. H. Moriones, A. Jimeno-Romero, B. Fischer and N. G. Bastus, et al., Assessment of iron oxide nanoparticle ecotoxicity on regeneration and homeostasis in the replacement model system Schmidtea mediterranea, ALTEX, 2019, 36(4), 583–596 Search PubMed.
- C. Peng, W. Zhang, H. Gao, Y. Li, X. Tong and K. Li, et al., Behavior and Potential Impacts of Metal-Based Engineered Nanoparticles in Aquatic Environments, Nanomaterials, 2017, 7(1), 21 CrossRef.
- M. Bhuvaneshwari, B. Sagar, S. Doshi, N. Chandrasekaran and A. Mukherjee, Comparative study on toxicity of ZnO and TiO 2 nanoparticles on Artemia salina: effect of pre-UV-A and visible light irradiation, Environ. Sci. Pollut. Res., 2017, 24, 5633–5646 CrossRef CAS.
- Z. Clemente, V. Castro, M. Moura, C. Jonsson and L. Fraceto, Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions, Aquat. Toxicol., 2014, 147, 129–139 CrossRef CAS.
- H. Ma, L. K. Wallis, S. Diamond, S. Li, J. Canas-Carrell and A. Parra, Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution, Environ. Pollut., 2014, 193, 165–172 CrossRef CAS PubMed.
- M. Erhayem and M. Sohn, Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter, Sci. Total Environ., 2014, 468, 249–257 CrossRef.
- X. Wang, S. Tao and B. Xing, Sorption and competition of aromatic compounds and humic acid on multiwalled carbon nanotubes, Environ. Sci. Technol., 2009, 43(16), 6214–6219 CrossRef CAS PubMed.
- K. Yang, D. Lin and B. Xing, Interactions of humic acid with nanosized inorganic oxides, Langmuir, 2009, 25(6), 3571–3576 CrossRef CAS PubMed.
- J. Gao, S. Youn, A. Hovsepyan, V. L. Llaneza, Y. Wang and G. Bitton, et al., Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: effects of water chemical composition, Environ. Sci. Technol., 2009, 43(9), 3322–3328 CrossRef CAS PubMed.
- R. Grillo, A. H. Rosa and L. F. Fraceto, Engineered nanoparticles and organic matter: a review of the state-of-the-art, Chemosphere, 2015, 119, 608–619 CrossRef CAS PubMed.
- S. P. Yang, O. Bar-Ilan, R. E. Peterson, W. Heideman, R. J. Hamers and J. A. Pedersen, Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish, Environ. Sci. Technol., 2013, 47(9), 4718–4725 CrossRef CAS.
- S. Noventa, D. Rowe and T. Galloway, Mitigating effect of organic matter on the in vivo toxicity of metal oxide nanoparticles in the marine environment, Environ. Sci.: Nano, 2018, 5(7), 1764–1777 RSC.
- Y. Zhang, T. A. Blewett, A. L. Val and G. G. Goss, UV-induced toxicity of cerium oxide nanoparticles (CeO 2 NPs) and the protective properties of natural organic matter (NOM) from the Rio Negro Amazon River, Environ. Sci.: Nano, 2018, 5(2), 476–486 RSC.
- S. M. Kteeba, H. I. El-Adawi, O. A. El-Rayis, A. E. El-Ghobashy, J. L. Schuld and K. R. Svoboda, et al., Zinc oxide nanoparticle toxicity in embryonic zebrafish: Mitigation with different natural organic matter, Environ. Pollut., 2017, 230, 1125–1140 CrossRef CAS PubMed.
- F. Seitz, R. R. Rosenfeldt, K. Storm, G. Metreveli, G. E. Schaumann and R. Schulz, et al., Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna, Ecotoxicol. Environ. Saf., 2015, 111, 263–270 CrossRef CAS PubMed.
- X. Liu, X. Jin, B. Cao and C. Y. Tang, Bactericidal activity of silver nanoparticles in environmentally relevant freshwater matrices: influences of organic matter and chelating agent, J. Environ. Chem. Eng., 2014, 2(1), 525–531 CrossRef CAS.
- S. Gehlout, Ecosafety Assessment of Iron Oxide Nanofertilizers, Doctoral dissertation, Deakin University, 2022 Search PubMed.
- A. Bedi, B. R. Singh, S. K. Deshmukh, N. Aggarwal, C. J. Barrow and A. Adholeya, Development of a novel myconanomining approach for the recovery of agriculturally important elements from jarosite waste, J. Environ. Sci., 2018, 67, 356–367 CrossRef CAS.
- O. Bar-Ilan, R. M. Albrecht, V. E. Fako and D. Y. Furgeson, Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos, Small, 2009, 5(16), 1897–1910 CrossRef CAS PubMed.
- J. E. Choi, S. Kim, J. H. Ahn, P. Youn, J. S. Kang and K. Park, et al., Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish, Aquat. Toxicol., 2010, 100(2), 151–159 CrossRef CAS PubMed.
- P. Asharani, Y. L. Wu, Z. Gong and S. Valiyaveettil, Toxicity of silver nanoparticles in zebrafish models, Nanotechnology, 2008, 19(25), 255102 CrossRef CAS PubMed.
- T. Horvat, M. Kalafatić, N. Kopjar and G. Kovačević, Toxicity testing of herbicide norflurazon on an aquatic bioindicator species–the planarian Polycelis felina (Daly.), Aquat. Toxicol., 2005, 73(4), 342–352 CrossRef CAS PubMed.
- A. Priyam, P. P. Singh, L. O. Afonso and A. G. Schultz, Abiotic factors and aging alter the physicochemical characteristics and toxicity of Phosphorus nanomaterials to zebrafish embryos, NanoImpact, 2022, 25, 100387 CrossRef CAS.
- K. Kanjana and S. Prasarnpun, Toxicity of nano-Zinc Oxide on Planarians (Dugesia japonica) with Neurobehavioral endpoints, NU. Int. J. Sci., 2018, 15(2), 76–88 Search PubMed.
- M. Žemberyová, J. Bartekova and I. Hagarova, The utilization of modified BCR three-step sequential extraction procedure for the fractionation of Cd, Cr, Cu, Ni, Pb and Zn in soil reference materials of different origins, Talanta, 2006, 70(5), 973–978 CrossRef PubMed.
- Q. Mu, G. Jiang, L. Chen, H. Zhou, D. Fourches and A. Tropsha, et al., Chemical basis of interactions between engineered nanoparticles and biological systems, Chem. Rev., 2014, 114(15), 7740–7781 CrossRef CAS.
- J. Piella, N. G. Bastús and V. Puntes, Modeling the Optical Responses of Noble Metal Nanoparticles Subjected to Physicochemical Transformations in Physiological Environments: Aggregation, Dissolution and Oxidation, Z. Phys. Chem., 2017, 231(1), 33–50 CrossRef CAS.
- B. Reidy, A. Haase, A. Luch, K. A. Dawson and I. Lynch, Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications, Materials, 2013, 6(6), 2295–2350 CrossRef CAS.
- S. P. Schwaminger, K. Schwarzenberger, J. Gatzemeier, Z. Lei and K. Eckert, Magnetically Induced Aggregation of Iron Oxide Nanoparticles for Carrier Flotation Strategies, ACS Appl. Mater. Interfaces, 2021, 13(17), 20830–20844 CrossRef CAS.
- L. Chekli, S. Phuntsho, M. Roy, E. Lombi, E. Donner and H. K. Shon, Assessing the aggregation behaviour of iron oxide nanoparticles under relevant environmental conditions using a multi-method approach, Water Res., 2013, 47(13), 4585–4599 CrossRef CAS PubMed.
- I. Römer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests, J. Chromatogr. A, 2011, 1218(27), 4226–4233 CrossRef PubMed.
- M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall and J. R. Lead, Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter, Environ. Toxicol. Chem., 2008, 27(9), 1875–1882 CrossRef CAS PubMed.
- M. Baalousha, Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter, Sci. Total Environ., 2009, 407(6), 2093–2101 CrossRef CAS.
- J. Zhang, M. Wages, S. B. Cox, J. D. Maul, Y. Li and M. Barnes, et al., Effect of titanium dioxide nanomaterials and ultraviolet light coexposure on African clawed frogs (Xenopus laevis), Environ. Toxicol. Chem., 2012, 31(1), 176–183 CrossRef CAS.
- J. Sun, L.-H. Guo, H. Zhang and L. Zhao, UV irradiation induced transformation of TiO2 nanoparticles in water: aggregation and photoreactivity, Environ. Sci. Technol., 2014, 48(20), 11962–11968 CrossRef CAS.
- J. M. Gorham, R. I. MacCuspie, K. L. Klein, D. H. Fairbrother and R. D. Holbrook, UV-induced photochemical transformations of citrate-capped silver nanoparticle suspensions, J. Nanopart. Res., 2012, 14, 1–16 CrossRef.
- T. P. Dasari and H.-M. Hwang, The effect of humic acids on the cytotoxicity of silver nanoparticles to a natural aquatic bacterial assemblage, Sci. Total Environ., 2010, 408(23), 5817–5823 CrossRef CAS.
- K. Kansara, A. Paruthi, S. K. Misra, A. S. Karakoti and A. Kumar, Montmorillonite clay and humic acid modulate the behavior of copper oxide nanoparticles in aqueous environment and induces developmental defects in zebrafish embryo, Environ. Pollut., 2019, 255(Pt 2), 113313 CrossRef CAS.
- A. M. Wormington, J. Coral, M. M. Alloy, C. L. Delmare, C. M. Mansfield and S. J. Klaine, et al., Effect of natural organic matter on the photo-induced toxicity of titanium dioxide nanoparticles, Environ. Toxicol. Chem., 2017, 36(6), 1661–1666 CrossRef CAS.
- V. A. Velintine, B. S. Wee, E. K. Droepenu, S. F. Chin and K. Y. Kok, Effects of humic acid and natural sunlight irradiation on the behaviour of zinc oxide nanoparticles in the aqueous environment, Biointerface Res. Appl. Chem., 2020, 11(4), 11256–11271 Search PubMed.
- L. Natarajan, M. A. Jenifer and A. Mukherjee, Eco-corona formation on the nanomaterials in the aquatic systems lessens their toxic impact: A comprehensive review, Environ. Res., 2021, 194, 110669 CrossRef CAS.
- C. Xie, X. Li, Z. Guo, Y. Dong, S. Zhang and A. Li, et al., Graphene oxide disruption of homeostasis and regeneration processes in freshwater planarian Dugesia japonica via intracellular redox deviation and apoptosis, Ecotoxicol. Environ. Saf., 2023, 249, 114431 CrossRef CAS PubMed.
- Z. Sun, E. Sokolova, J. E. Brittain, S. J. Saltveit, S. Rauch and S. Meland, Impact of environmental factors on aquatic biodiversity in roadside stormwater ponds, Sci. Rep., 2019, 9(1), 5994 CrossRef PubMed.
- J. Kirch, A. Schneider, B. Abou, A. Hopf, U. F. Schaefer and M. Schneider, et al., Optical tweezers reveal relationship between microstructure and nanoparticle penetration of pulmonary mucus, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(45), 18355–18360 CrossRef CAS.
- R. Behra, L. Sigg, M. J. Clift, F. Herzog, M. Minghetti and B. Johnston, et al., Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective, J. R. Soc., Interface, 2013, 10(87), 20130396 CrossRef.
- M. Vila-Farre, A. Rozanski, M. Ivankovic, J. Cleland, J. N. Brand and F. Thalen, et al., Evolutionary dynamics of whole-body regeneration across planarian flatworms, Nat. Ecol. Evol., 2023, 7(12), 2108–2124 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00449c |
| This journal is © The Royal Society of Chemistry 2025 |