Open Access Article
Open Access ArticleAre pure hydrocarbons the future of host materials for blue phosphorescent organic light-emitting diodes?
Cyril
Poriel
 *a,
Joëlle
Rault-Berthelot
*a,
Joëlle
Rault-Berthelot
 a and
Zuo-Quan
Jiang
a and
Zuo-Quan
Jiang
 b
b
aUniv Rennes, CNRS, ISCR-6226, F-35000 Rennes, France
bInstitute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou, Jiangsu 215123, China. E-mail: cyril.poriel@univ-rennes1.fr
First published on 10th March 2022
Abstract
The fantastic development of phosphorescent organic light-emitting diodes (PhOLEDs) has been undoubtedly driven by the molecular design of high-efficiency host materials for red, green and blue phosphors. Fine tuning the electronic and physical properties of the host materials has allowed very high External Quantum Efficiencies (EQEs) to be reached, reported nowadays above 30% for the three colours. The most used molecular design strategy consists of judiciously assembling small functional units to maintain a high triplet energy level (ET), essential to ensure efficient energy transfer from the host to the guest phosphor. To adjust the molecular orbital energy levels and the charge transport, these functional units display electron-donating (for hole transport) and electron-accepting (for electron transport) capabilities. Hundreds of host materials have been reported in the literature since the discovery of PhOLEDs in 1998. However, they all have a common characteristic: they incorporate heteroatoms. However, although these functional units are highly beneficial for the device performance, several works have highlighted their potential instability in the device. As the stability of blue-emitting PhOLEDs is a central concern in the field, finding alternatives to heteroatom-based hosts is a mandatory step. In recent years, pure hydrocarbon (PHC) materials, only built with carbon and hydrogen atoms, have shown real potential for the next generation of PhOLEDs. In the light of the literature, it appears nowadays that the PHC design strategy is very promising for the future development of the OLED industry as a high-performance and low-cost option. This is the purpose of the present Chemistry Frontiers.
Introduction
Designing high efficiency host materials for the emerging technologies of Phosphorescent Organic Light-Emitting Diodes (PhOLEDs) has been, since their discovery in 1998, one of the main driving forces of the field.1 The EMitting Layer (EML) of a PhOLED consists of a triplet emitter (Guest) dispersed into an appropriate Organic Semi-Conductor (Host). This technique allows 100% internal quantum efficiency to be reached by recovering both singlet and triplet excitons.1 Bipolar hosts2,3 constructed on the assembly of electron-rich, and electron-poor units, see examples selected among the most efficient materials in each colour in Chart 1,4–10 have appeared as the most efficient host materials. Over the years, their designs have been finely tuned in order to reach very high PhOLED performance. Nowadays, the design techniques are rather well-known and the most efficient host materials have achieved very high external quantum efficiency (EQE) for red, green and even blue PhOLEDs (>30%).3–10 One of the advantages of donor/acceptor host materials is their capability to efficiently transport both electrons and holes. For example, the SPA-2,7-F(POPh2)2 host, reported in 2020, displays rather well-balanced mobilities between holes (8.2 × 10−6 cm2 V−1 s−1) and electrons (2 × 10−4 cm2 V−1 s−1),8,11 which favour a good charge recombination in the centre of the EML of the device. Thanks to this property, SPA-2,7-F(POPh2)2 has been efficiently incorporated in simplified single-layer PhOLEDs (EQE of 18%), in which the additional functional transporting layers have been removed.8,11 | ||
| Chart 1 Selected examples of state of the art heteroatom-based host materials.4–10 | ||
Although very high performances have been reached, PhOLED technology still suffers from many issues, and the stability of blue PhOLEDs is one of the most important. Reaching high efficiency and stable blue PhOLEDs is undoubtedly the next important barrier to lift towards their industrial exploitation. From a general point of view, blue OLEDs (either fluorescent, phosphorescent, TADF or hyperfluorescent)12–15 have always been the weakest link of the technology both in terms of efficiency (due to the large gap of blue emitting materials) and stability.3,16–22 The instability of blue PhOLEDs has been the subject of intense research.23–25 It is now widely known that FIrpic, the most used blue phosphor, is involved in the PhOLED degradation processes.24 However, the host material is also an important piece of the puzzle and also involved, at least partially, in the degradation processes. Thus, heteroatom-based host materials, despite their very high performances, present several drawbacks.
First, their molecular structures can be complicated due to the assembly of different donor and acceptor fragments, which increases the synthetic complexity, the environmental footprint and the production costs. As different building units are used, many issues can occur. For example, the diazafluorene building block is a good electron accepting fragment but appears to be difficult to handle due to chelating and solubility issues.26,27 Quinolinophenothiazine is a recently discovered electron-rich fragment built on a phenylacridine core bridged by a sulphur atom.28 This fragment has recently provided very high performance in simplified PhOLEDs.29 Despite a high HOMO level and stable radical cation species,28,29 this fragment suffers from the inversion of the nitrogen atom leading to diastereoisomeric features.30 It is obvious that for the next step of the OLED technology, very simple molecular structures, which can be easily and cleanly synthesized, should be considered. This is a key consideration for the future to provide a new generation of electronics, essential for the ecological transition.
Second, regarding the stability, it has been shown that the C–N, C–P and C–S bonds, particularly in their excited states, can be easily broken by the energy of excitons formed in blue PhOLEDs (which is around 3 eV).31–33 Indeed, the dissociation energies of the C–N, C–P or C–S bonds in common building units used in host materials lies in this range.33 In 2013, König and coworkers have reported the key role played by the bond dissociation energies in the chemical degradations in OLEDs (Exciton induced degradation process).25 Similarly, Bredas and coworkers have explored the bond dissociation processes in arylsulfones and arylphosphine oxides, two famous building units of host materials, and have shown that C–S and C–P bonds are significantly more vulnerable to dissociation in their first triplet state than in the ground state.34
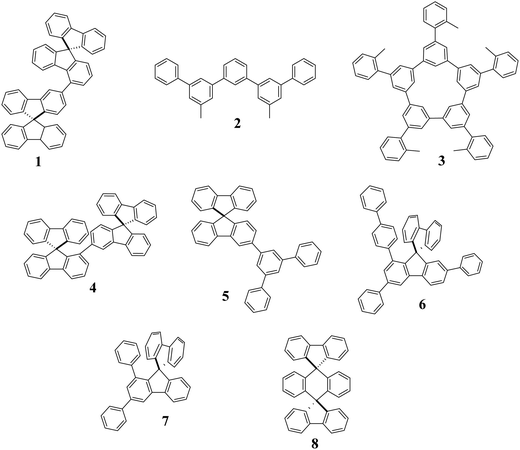
Thus, pure hydrocarbons (PHC), which are molecules free of heteroatoms, only built with carbon and hydrogen atoms have emerged as a possible solution to increase the stability of blue PhOLEDs (see the most efficient materials reported to date in Fig. 1).35 PHCs only use phenyl rings as a building unit, which is one of the most inert and stable molecular fragments. Therefore, they do not possess any weak heteroatom-based bonds and can be, most of the time, very easily synthesized. The first example of PHC in a PhOLED was reported in 2005 (Fig. 2), using a low ET red phosphor as the emitter.36
Some key dates in the history of PHC are presented in Fig. 2. However, elaborating efficient host materials with only phenyl rings appeared, at the beginning, as a very difficult task. Nowadays, this barrier has been lifted and in 15 years, the PHC design to host phosphors in PhOLEDs has become a credible alternative to bipolar hosts containing heteroatoms. Indeed, year after year, the performances of PHC-based devices have increased thanks to the different design tactics elaborated by chemists and in 2020, it was shown that PHC hosts can overpass heteroatom-based host materials (see below).37
So, how have PHCs become efficient host materials in PhOLEDs and what are the most efficient designs used to date? The first step was to judiciously assemble phenyl rings to reach high ET materials. In this context, an important date in the development of PHC hosts was 2011, when the electronic decoupling using meta linkage was introduced in PHCs for PhOLEDs.38 This linkage has appeared as an efficient molecular tool to disrupt the conjugation of a π-system. This π-conjugation disruption is essential to keep a high ET, which is a key property to host a blue phosphor. Many PHC host materials were then synthesised with the meta linkage as a key caracteristic.39–46 Despite the PhOLED performances not always being very high (EQE of blue PhOLED below 16%), all these works have significantly contributed to the development of PHC hosts and have undoubtedly opened the way to the high performance reported nowadays. In 2015, Jiang, Liao and their coworkers reported the first PHC-based blue PhOLEDs (1) with an EQE above 20%, clearly opening new perspectives for this class of materials.47 Very high efficiency white-emitting PhOLEDs (combination of FIrpic blue emitter and PO-01 yellow emitter) were also fabricated using 1 as a host, displaying a very high EQE of 25.3%.
In 2016, a very high EQE of 24.8% was also obtained by Isobe and coworkers with PHC 3 in a simple green-emitting two-region phosphorescent device (structure: ITO/PEDOT:PSS/non-doped Host/Host:Ir(ppy)3 6%)/Cs/Al). Interestingly, they have highlighted the significant differences between linear (such as 2) and cyclic (such as 3) meta-linked PHCs when used as a host for green phosphors.44–46 In 2020, the same group reported an impressive value of 21.4% with 2 as a host in green emitting simplified single-layer PhOLEDs.44 This performance is the highest reported to date for a green single-layer-PhOLED (including heteroatoms-based hosts). As single-layer devices2,8,11,20,29,48 only built with electrodes and EML are nowadays more and more appealing for the future of OLED technology, this work also shows the real potential of PHC materials in this emerging technology. For more details on SL-PhOLEDs, see the recent review published in 2021.2
In the elaboration of high efficiency PHC host materials, the building units used are of key importance. They should not only allow restriction of the π-conjugation to keep a high ET but they should also possess very good thermal and morphological stabilities (high decomposition temperature Td and high glass transition temperature Tg). Regarding the latter, the spirobifluorene (SBF) fragment is a 3D molecular scaffold, widely known in organic electronics for its capacity to highly increase Td/Tg.40,49,50 The impact of the SBF substitution pattern on the electronic properties and particularly on the ET is far more recent but has been an important step in the development of high efficiency PHCs as hosts.40 Thus, the C1-substituted SBF scaffold, reported in 2017,51 appeared as an important building block to efficiently restrict the π-conjugation extension thanks to its meta linkage keeping in the meantime excellent thermal and morphological properties owing to its spiro bridge.40 In 2019, the highly twisted SBF dimer 4 constructed on the C1-substituted SBF scaffold was incorporated as a host in blue FIrpic PhOLEDs, 4 displaying an impressive EQE of 22.9%, which was, at that time, the highest reported in the literature for a PHC host.52 This device also displayed low turn-on voltages, below 3 V. As turn-on voltage is one of the main issues in blue emitting devices, this work has shown that the PHC strategy can be compatible with a good charge injection. Despite this performance being very high and promising for the future of PHCs, it was still incomparable with the best heteroatom-based hosts in blue, green, and red OLEDs (these materials adapted for the three phosphors are called ‘universal’).22–25 In 2020, the full potential of PHC host materials has been released as the simple PHC host 5, only constructed on a C3-SBF terphenyl assembly, has overpassed the performance reached by the best universal heteroatom-based hosts.9,53 This PHC has the huge advantage of being very easily synthesized in a single step with a very high yield at the multi-gram scale. This is undoubtedly of key importance for future industrialization.
Thus, PHC hosts hold nowadays two record performances in the field of PhOLEDs, the most efficient universal host (5)37 and the most efficient host (2) in a green-emitting single-layer device.44 Despite the state of the art performances in red, green and blue PhOLEDs still being held by bipolar materials, the performances of PHC hosts are getting closer and closer and this is undoubtedly an important feature. However, their potential in terms of stability is difficult to evaluate as there is, to the best of our knowledge, no stability study published to date with PhOLEDs. There is, nevertheless, one study published on the stability of a PHC-based device using a TADF emitter, which has shown very promising results (see below).47 Such studies will be highly relevant in the coming years.
As the performances are now very high, the interest towards PHC continues to rise and improving the synthesis efficiency is an important step. In early 2021, Lan, You and their coworkers54 reported poly-substituted SBF-based PHCs as hosts for blue PhOLEDs (6 and 7, Fig. 1). They reported the first examples of diarylation/annulation of benzoic acids via an iridium catalyst system, providing a step-economic and efficient pathway to 1-aryl, 1,3-diaryl, 1,7-diaryl and 1,3,7-triaryl SBFs. This work not only shows the efficiency of the C–H activation for the development of PHC materials but also allows poly-substituted SBFs to be reached, which are very difficult to synthesize. Thanks to the poly-substitution, 6 displays excellent thermal properties (Tg of 140 °C) and a very high EQE of 27.3% when hosting a red phosphor. A very simple modification of the starting material leads to the 1,3-disubstituted SBF 7, which displays a high ET of 2.83 eV and a good EQE of 18.6% when incorporated as a host in a blue PhOLED. Thus, the C–H activation will surely be an efficient tool in the future design of efficient PHC-based materials.
In terms of molecular design, increasing the ET of PHCs is undoubtedly a key direction for the future in order to host deep blue phosphors, which are still missing. To reach this goal, molecular adjustments should be performed such as those very recently performed by Thompson and coworkers.55 They have designed the PHC host 8 constructed on two fluorenyl units spirolinked to a dihydroanthracene core. The structural particularity of this PHC host is linked to the fact that there is no pending substituent, which allows it to display a very high ET of 2.92 eV and a very wide HOMO/LUMO difference of above 5 eV. The PhOLED incorporates a barely used blue phosphor, namely Fac-tris(N,N-di-p-tolyl-pyrizinoimidazol-2-yl)iridium(III) Ir(tpz)3, known to be more stable than FIrpic, and displays a high EQE of 18% and a low turn on voltage (2.9 V). Removing all the pending substituents is clearly a right way to increase ET and a very interesting strategy for the future. However, it should be mentioned that this strategy has already been used notably with SBF, with nevertheless lower performance.56 The importance of the substitution in the resulting PhOLED performance is thus not easy to forecast. This also highlights the central role played by the device engineering to reach high performance blue PhOLEDs. However, a judicious substitution allows to finely tune the physical and electronic properties of a PHC scaffold, without strongly decreasing the ET.57 Such a compromise should be considered.
Conclusions and outlooks
PHC hosts, free of heteroatoms, represent nowadays a key evolution in the OLED technology. They can be a possible solution to the blue PhOLED instability and their simple structure and synthesis can be a solution to the production costs. The design of donor/acceptor host materials has attracted tremendous interest for the last 20 years but the interest towards PHC based materials is more recent. Despite the first example being published in 2005, PHC hosts did not strongly influence the scientific community at the beginning, surely because bipolar materials, notably based on the widely known carbazole or phosphine oxide functional units, were far more efficient. However, year after year, the molecular simplicity and easy synthesis of PHCs have attracted more and more attention from research groups involved in the design of host materials. As nowadays some PHC hosts have shown very high potential, they can be key actors for the future of PhOLED technology.What are the next challenges that PHC materials will have to face?
First, as the blue PhOLED technology is still the weakest link of the technology, improving the blue PhOLED performance with PHC hosts remains an important challenge. More importantly, in almost all the examples reported in the literature, the blue-greenish FIrpic emitter is used. However, FIrpic emission is not in the deep blue region (CIE of ca. 0.15, 0.38) and other phosphors emitting at short wavelengths (deep blue and violet) should be investigated in the future. However, such emitters are not highly developed in the literature, which significantly increases the scientific challenge. For example, phosphor FIr6 possesses an emission at shorter wavelengths (CIE of ca. 0.15, 0.32) and has only been tested in a very few instances with PHC hosts. This constitutes an interesting direction for the future of PHCs. However, the CIEs of FIr6 are still far in the deep blue region and other phosphors should be developed. To host such deep blue phosphors, the ET of PHCs should be increased. Thus, the next challenge of PHC hosts will be to increase their ET to efficiently host a new generation of deep blue phosphors. For that, novel molecular design approaches should also be developed, with for example no pending substituent. This strategy has recently been developed by Thompson and coworkers55 and appears promising. The combination of deep blue phosphors and PHC hosts is surely of great interest for OLED technology.The stability of the blue emitters should also be significantly improved. As FIrpic is unstable and degrades through the loss of the picolinate ancillary ligand, finding a stable alternative to this widely used phosphor is a key requirement.24 Ir(tpz)3, a cyclometalated N-heterocyclic carbene, more stable than traditional Ir complexes with datively bound nitrogen ligands such as FIrpic58 starts to show great potential. This type of Ir complex appears interesting for the future of this technology.59 However, the CIE coordinates of this phosphorescent emitter (0.17–19, 0.37–0.40)55 are roughly similar to those of FIrpic, being therefore inadequate for deep blue emission.
With the new generation of PHC hosts, it will also be important to precisely investigate the stability of the corresponding devices, which is not systematically performed in the PhOLED field. This will be key in the development of PHC-based PhOLEDs. Thus, in the blue PhOLED technology, the combination of PHC hosts with a more stable Ir complex, such as (Ir(tpz)3), appears as a promising strategy. Another key perspective of PHC-based PhOLEDs lies in white emitting PHC-based devices, which are almost absent from the literature. As far as we know, there is only one example reported to date.47 As white light emission can be obtained by the combination, in a single EML, of two complementary emissions (i.e. association of a blue and a yellow phosphorescent emitter), a high ET PHC host could be, in principle, used. This is an interesting direction for the production of low-cost white-emitting devices for lighting applications.
Finally, the potential of PHC hosts could be extended to other generations of OLED, such as TADF OLEDs. Indeed, in 2015, Jiang and coworkers showed that PHCs can also efficiently host green TADF emitters such as (2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile, 4CzIPN) with a very high EQE of 22.3%.47 Beside the very high EQE reached, the group has shown that the device lifetime was also significantly improved compared to that using the well-known 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP) as a host. This has been an important step but remains nevertheless, to date, one of the rare stability studies reported with PHC hosts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank all the researchers involved in the PHC project in our groups (Rennes and Soochow). Dr J. F Bergamini is warmly acknowledged for the graphical abstract design.References
- M. A. Baldo, D. F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices, Nature, 1998, 395, 151 Search PubMed
.
- C. Poriel and J. Rault-Berthelot, Designing Host Materials for the Emissive Layer of Single-Layer Phosphorescent Organic Light-Emitting Diodes: Toward Simplified Organic Devices, Adv. Funct. Mater., 2021, 31, 2010547 Search PubMed
.
- Y. Wang, J. H. Yun, L. Wang and J. Y. Lee, High Triplet Energy Hosts for Blue Organic Light-Emitting Diodes, Adv. Funct. Mater., 2020, 31, 2008332 Search PubMed
.
- D. Li, J. Li, D. Liu, W. Li, C.-L. Ko, W.-Y. Hung and C. Duan, Highly Efficient Simple-Structure Sky-Blue Organic Light-Emitting Diode Using a Bicarbazole/Cyanopyridine Bipolar Host, ACS Appl. Mater. Interfaces, 2021, 13, 13459 Search PubMed
.
- Q.-L. Xu, X. Liang, S. Zhang, Y.-M. Jing, X. Liu, G.-Z. Lu, Y.-X. Zheng and J.-L. Zuo, Efficient OLEDs with low efficiency roll-off using iridium complexes possessing good electron mobility, J. Mater. Chem. C, 2015, 3, 3694 Search PubMed
.
- W. Song, Q. Xu, J. Zhu, Y. Chen, H. Mu, J. Huang and J. Su, Imidazo[1,2-b]pyridazine as Building Blocks for Host Materials for High-Performance Red-Phosphorescent Organic Light-Emitting Devices, ACS Appl. Mater. Interfaces, 2020, 12, 19701 Search PubMed
.
- X. Tang, X.-Y. Liu, Y. Yuan, Y.-J. Wang, H.-C. Li, Z.-Q. Jiang and L.-S. Liao, High-Efficiency White Organic Light-Emitting Diodes Integrating Gradient Exciplex Allocation System and Novel D-Spiro-A Materials, ACS Appl. Mater. Interfaces, 2018, 10, 29840 Search PubMed
.
- F. Lucas, C. Quinton, S. Fall, T. Heiser, D. Tondelier, B. Geffroy, N. Leclerc, J. Rault-Berthelot and C. Poriel, Universal host materials for red, green and blue high-efficiency single-layer phosphorescent organic light-emitting diodes, J. Mater. Chem. C, 2020, 8, 16354 Search PubMed
.
- W.-C. Chen, Y. Yuan, Z.-L. Zhu, Z.-Q. Jiang, S.-J. Su, L.-S. Liao and C.-S. Lee, De novo design of D–σ–A molecules as universal hosts for monochrome and white phosphorescent organic light-emitting diodes, Chem. Sci., 2018, 9, 4062 Search PubMed
.
- K. Udagawa, H. Sasabe, C. Cai and J. Kido, Low-Driving-Voltage Blue Phosphorescent Organic Light-Emitting Devices with External Quantum Efficiency of 30%, Adv. Mater., 2014, 26, 5062 Search PubMed
.
- F. Lucas, O. A. Ibraikulov, C. Quinton, L. Sicard, T. Heiser, D. Tondelier, B. Geffroy, N. Leclerc, J. Rault-Berthelot and C. Poriel, Spirophenylacridine-2,7-(diphenylphosphineoxide)-fluorene: A Bipolar Host for High-Efficiency Single-Layer Blue Phosphorescent Organic Light-Emitting Diodes, Adv. Opt. Mater., 2020, 8, 1901225 Search PubMed
.
- A. Monkman, Why Do We Still Need a Stable Long Lifetime Deep Blue OLED Emitter?, ACS Appl. Mater. Interfaces, 2022 DOI:10.1021/acsami.1c09189
.
- C.-Y. Chan, M. Tanaka, Y.-T. Lee, Y.-W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission, Nat. Photonics, 2021, 15, 203 Search PubMed
.
- C. Adachi, 37.1: Invited Paper: Third Generation OLED by Hyperfluorescence, SID Int. Symp. Dig. Tech. Pap., 2013, 44, 513 Search PubMed
.
- H. Abroshan, V. Coropceanu and J.-L. Brédas, Hyperfluorescence-Based Emission in Purely Organic Materials: Suppression of Energy-Loss Mechanisms via Alignment of Triplet Excited States, ACS Mater. Lett., 2020, 2, 1412 Search PubMed
.
- J.-H. Lee, C.-H. Chen, P.-H. Lee, H.-Y. Lin, M.-K. Leung, T.-L. Chiu and C.-F. Lin, Blue organic light-emitting diodes: current status, challenges, and future outlook, J. Mater. Chem. C, 2019, 7, 5874 Search PubMed
.
- T.-T. Bui, F. Goubard, M. Ibrahim-Ouali, D. Gigmes and F. Dumur, Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs), Beilstein J. Org. Chem., 2018, 14, 282 Search PubMed
.
- Y. Im, S. Y. Byun, J. H. Kim, D. R. Lee, C. S. Oh, K. S. Yook and J. Y. Lee, Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes, Adv. Funct. Mater., 2017, 27, 1603007 Search PubMed
.
- K. S. Yook and J. Y. Lee, Organic Materials for Deep Blue Phosphorescent Organic Light-Emitting Diodes, Adv. Mater., 2012, 24, 3169 Search PubMed
.
- C. Poriel and J. Rault-Berthelot, Blue Single-Layer Organic Light-Emitting Diodes Using Fluorescent Materials: A Molecular Design View Point, Adv. Funct. Mater., 2020, 30, 1910040 Search PubMed
.
- R. Mertens, The OLED Handbook: A Guide to OLED Technology, Industry & Market. 2019 edition: 2019.
- Y. Li, J.-Y. Liu, Y.-D. Zhao and Y.-C. Cao, Recent advancements of high efficient donor–acceptor type blue small molecule applied for OLEDs, Mater. Today, 2017, 20, 258 Search PubMed
.
- W. Song and J. Y. Lee, Degradation Mechanism and Lifetime Improvement Strategy for Blue Phosphorescent Organic Light-Emitting Diodes, Adv. Opt. Mater., 2017, 5, 1600901 Search PubMed
.
- M. Penconi, M. Cazzaniga, W. Panzeri, A. Mele, F. Cargnoni, D. Ceresoli and A. Bossi, Unraveling the Degradation Mechanism in FIrpic-Based Blue OLEDs: II. Trap and Detect Molecules at the Interfaces, Chem. Mater., 2019, 31, 2277 Search PubMed
.
- S. Schmidbauer, A. Hohenleutner and B. König, Chemical Degradation in Organic Light-Emitting Devices: Mechanisms and Implications for the Design of New Materials, Adv. Mater., 2013, 25, 2114 Search PubMed
.
- M. Romain, D. Tondelier, O. Jeannin, B. Geffroy, J. Rault-Berthelot and C. Poriel, Properties modulation of organic semi-conductors based on a donor-spiro-acceptor (D-spiro-A) molecular design: new host materials for efficient sky-blue PhOLEDs, J. Mater. Chem. C, 2015, 3, 97010 Search PubMed
.
- S. Thiery, D. Tondelier, B. Geffroy, O. Jeannin, J. Rault-Berthelot and C. Poriel, Modulation of the Physicochemical Properties of Donor–Spiro–Acceptor Derivatives through Donor Unit Planarisation: Phenylacridine versus Indoloacridine—New Hosts for Green and Blue Phosphorescent Organic Light-Emitting Diodes (PhOLEDs), Chem. – Eur. J., 2016, 22, 10136 Search PubMed
.
- C. Poriel, J. Rault-Berthelot, S. Thiery, C. Quinton, O. Jeannin, U. Biapo, B. Geffroy and D. Tondelier, 9H-Quinolino[3,2,1-k]phenothiazine: A New Electron-Rich Fragment for Organic Electronics, Chem. – Eur. J., 2016, 22, 17930 Search PubMed
.
- F. Lucas, D. Tondelier, B. Geffroy, T. Heiser, O. A. Ibraikulov, C. Quinton, C. Brouillac, N. Leclerc, J. Rault-Berthelot and C. Poriel, Quinolinophenothiazine as Electron Rich Fragment for RGB Single-Layer Phosphorescent Organic Light-Emitting Diodes, Mater. Chem. Front., 2021, 5, 8066 Search PubMed
.
- C. Quinton, L. Sicard, O. Jeannin, N. Vanthuyne and C. Poriel, Confining Nitrogen Inversion to Yield Enantiopure Quinolino[3,2,1-k]Phenothiazine Derivatives, Adv. Funct. Mater., 2018, 28, 1803140 Search PubMed
.
- D. Y. Kondakov, W. C. Lenhart and W. F. Nichols, Operational degradation of organic light-emitting diodes: Mechanism and identification of chemical products, J. Appl. Phys., 2007, 101, 024512 Search PubMed
.
- N. Lin, J. Qiao, L. Duan, H. Li, L. Wang and Y. Qiu, Achilles Heels of Phosphine Oxide Materials for OLEDs: Chemical Stability and Degradation Mechanism of a Bipolar Phosphine Oxide/Carbazole Hybrid Host Material, J. Phys. Chem. C, 2012, 116, 19451 Search PubMed
.
- N. Lin, J. Qiao, L. Duan, L. Wang and Y. Qiu, Molecular Understanding of the Chemical Stability of Organic Materials for OLEDs: A Comparative Study on Sulfonyl, Phosphine-Oxide, and Carbonyl-Containing Host Materials, J. Phys. Chem. C, 2014, 118, 7569 Search PubMed
.
- H. Li, M. Hong, A. Scarpaci, X. He, C. Risko, J. S. Sears, S. Barlow, P. Winget, S. R. Marder, D. Kim and J.-L. Brédas, Chemical Stabilities of the Lowest Triplet State in Aryl Sulfones and Aryl Phosphine Oxides Relevant to OLED Applications, Chem. Mater., 2019, 31, 1507 Search PubMed
.
- C. Poriel and J. Rault-Berthelot, Pure hydrocarbons: An efficient molecular design strategy for the next generation of host materials for Phosphorescent Organic Light-Emitting Diodes, Acc. Mater. Res., 2022 DOI:10.1021/accountsmr.1c00263
.
- K.-T. Wong, Y.-L. Liao, Y.-T. Lin, H.-C. Su and C.-C. Wu, Spiro-Configured Bifluorenes:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Highly Efficient Emitter for UV Organic Light-Emitting Device and Host Material for Red Electrophosphorescence, Org. Lett., 2005, 7, 5131 Search PubMed
Highly Efficient Emitter for UV Organic Light-Emitting Device and Host Material for Red Electrophosphorescence, Org. Lett., 2005, 7, 5131 Search PubMed .
- Q. Wang, F. Lucas, C. Quinton, Y.-K. Qu, J. Rault-Berthelot, O. Jeannin, S.-Y. Yang, F.-C. Kong, S. Kumar, L.-S. Liao, C. Poriel and Z.-Q. Jiang, Evolution of pure hydrocarbon hosts: simpler structure, higher performance and universal application in RGB phosphorescent organic light-emitting diodes, Chem. Sci., 2020, 11, 4887 Search PubMed
.
- C. Poriel, R. Métivier, J. Rault-Berthelot, D. Thirion, F. Barrière and O. Jeannin, A robust pure hydrocarbon derivative based on the (2,1-b)-indenofluorenyl core with high triplet energy level, Chem. Commun., 2011, 47, 11703 Search PubMed
.
- M. Romain, S. Thiery, A. Shirinskaya, C. Declairieux, D. Tondelier, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel,
ortho-, meta-, and para-Dihydroindenofluorene Derivatives as Host Materials for Phosphorescent OLEDs, Angew. Chem., Int. Ed., 2015, 54, 1176 Search PubMed
.
- C. Poriel, L. Sicard and J. Rault-Berthelot, New generations of spirobifluorene regioisomers for organic electronics: tuning electronic properties with the substitution pattern, Chem. Commun., 2019, 55, 14238 Search PubMed
.
- S. Ye, Y. Liu, C.-A. Di, H. Xi, W. Wu, Y. Wen, K. Lu, C. Du, Y. Liu and G. Yu, Wide-Energy-Gap Host Materials for Blue Phosphorescent Organic Light-Emitting Diodes, Chem. Mater., 2009, 21, 1333 Search PubMed
.
- G. Tian, Y. Jiang, P. Wu, J. Huang, Q. Zou, Q. Wang, H. Mu and J. Su, Pure hydrocarbon host materials based on spirofluorene with excellent performances for green phosphorescent light-emitting devices, New J. Chem., 2016, 40, 9500 Search PubMed
.
- M. Zhuo, W. Sun, G. Liu, J. Wang, L. Guo, C. Liu, B. Mi, J. Song and Z. Gao, Pure aromatic hydrocarbons with rigid and bulky substituents as bipolar hosts for blue phosphorescent OLEDs, J. Mater. Chem. C, 2015, 3, 9137 Search PubMed
.
- A. Yoshii, Y. Onaka, K. Ikemoto, T. Izumi, S. Sato, H. Kita, H. Taka and H. Isobe, Acyclic, linear oligo-meta-phenylenes as multipotent base materials for highly efficient single-layer organic light-emitting devices, Chem. – Asian J., 2020, 15, 2181 Search PubMed
.
- J. Y. Xue, T. Izumi, A. Yoshii, K. Ikemoto, T. Koretsune, R. Akashi, R. Arita, H. Taka, H. Kita, S. Sato and H. Isobe, Aromatic hydrocarbon macrocycles for highly efficient organic light-emitting devices with single-layer architectures, Chem. Sci., 2016, 7, 896 Search PubMed
.
- K. Ikemoto, A. Yoshii, T. Izumi, H. Taka, H. Kita, J. Y. Xue, R. Kobayashi, S. Sato and H. Isobe, Modular Synthesis of Aromatic Hydrocarbon Macrocycles for Simplified, Single-Layer Organic Light-Emitting Devices, J. Org. Chem., 2016, 81, 662 Search PubMed
.
- L.-S. Cui, Y.-M. Xie, Y.-K. Wang, C. Zhong, Y.-L. Deng, X.-Y. Liu, Z.-Q. Jiang and L.-S. Liao, Pure Hydrocarbon Hosts for ≈100% Exciton Harvesting in Both Phosphorescent and Fluorescent Light-Emitting Devices, Adv. Mater., 2015, 27, 4213 Search PubMed
.
- S. Thiery, D. Tondelier, B. Geffroy, E. Jacques, M. Robin, R. Métivier, O. Jeannin, J. Rault-Berthelot and C. Poriel, Spirobifluorene-2,7-dicarbazole-4′-phosphine Oxide as Host for High-Performance Single-Layer Green Phosphorescent OLED Devices, Org. Lett., 2015, 17, 4682 Search PubMed
.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Spiro Compounds for Organic Optoelectronics, Chem. Rev., 2007, 107, 1011 Search PubMed
.
- S. Liu, D. Xia and M. Baumgarten, Rigidly-Fused Spiro-Conjugated π-Systems, ChemPlusChem, 2021, 21, 36 CrossRef PubMed
.
- L. Sicard, C. Quinton, J.-D. Peltier, D. Tondelier, B. Geffroy, U. Biapo, R. Métivier, O. Jeannin, J. Rault-Berthelot and C. Poriel, Spirobifluorene Regioisomerism: A Structure–Property Relationship Study, Chem. – Eur. J., 2017, 23, 7719 CrossRef CAS
.
- L. J. Sicard, H.-C. Li, Q. Wang, X.-Y. Liu, O. Jeannin, J. Rault-Berthelot, L.-S. Liao, Z.-Q. Jiang and C. Poriel, C1-Linked Spirobifluorene Dimers: Pure Hydrocarbon Hosts for High-Performance Blue Phosphorescent OLEDs, Angew. Chem., Int. Ed., 2019, 58, 3848 CrossRef CAS PubMed
.
- C.-C. Lai, M.-J. Huang, H.-H. Chou, C.-Y. Liao, P. Rajamalli and C.-H. Cheng, m-Indolocarbazole Derivative as a Universal Host Material for RGB and White Phosphorescent OLEDs, Adv. Funct. Mat., 2015, 25, 5548 CAS
.
- Y. Luo, Z. Liu, G. Yang, T. Wang, Z. Bin, J. Lan, D. Wu and J. You, Iridium(III)-Catalyzed Diarylation/Annulation of Benzoic Acids: Facile Access to Multi-Aryl Spirobifluorenes as Pure Hydrocarbon Hosts for High-Performance OLEDs, Angew. Chem., Int. Ed., 2021, 60, 18852 CrossRef CAS PubMed
.
- J. Ma, M. Idris, T. Y. Li, D. S. M. Ravinson, T. Fleetham, J. Kim, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Symmetric “Double Spiro” Wide Energy Gap Hosts for Blue Phosphorescent OLED Devices, Adv. Opt. Mater., 2022, 2101530 CrossRef CAS
.
- S. Thiery, D. Tondelier, C. Declairieux, G. Seo, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, 9,9′-Spirobifluorene and 4-phenyl-9,9′-spirobifluorene: pure hydrocarbon small molecules as hosts for efficient green and blue PhOLEDs, J. Mater. Chem. C, 2014, 2, 4156 RSC
.
- C. Poriel, C. Quinton, F. Lucas, J. Rault-Berthelot, Z.-Q. Jiang and O. Jeannin, Spirobifluorene Dimers: Understanding How The Molecular Assemblies Drive The Electronic Properties, Adv. Funct. Mater., 2021, 31(43), 2104980 CAS
.
- M. Idris, S. C. Kapper, A. C. Tadle, T. Batagoda, D. S. Muthiah Ravinson, O. Abimbola, P. I. Djurovich, J. Kim, C. Coburn, S. R. Forrest and M. E. Thompson, Blue Emissive fac/mer-Iridium(III) NHC Carbene Complexes and their Application in OLEDs, Adv. Opt.
Mater., 2021, 9, 2001994 CrossRef CAS
.
- X. Zhou and B. J. Powell, Nonradiative Decay and Stability of N-Heterocyclic Carbene Iridium(III) Complexes, Inorg. Chem., 2018, 57, 8881 CAS
.
| This journal is © the Partner Organisations 2022 |