Mechanofluorescent polymer/silsesquioxane composites based on tetraarylsuccinonitrile†
Fumika
Hoshino
,
Takahiro
Kosuge
,
Daisuke
Aoki
 and
Hideyuki
Otsuka
and
Hideyuki
Otsuka
 *
*
Department of Chemical Science and Engineering, Tokyo Institute of Technology, 2-12-1 S1-6 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: otsuka@polymer.titech.ac.jp
First published on 6th November 2019
Abstract
Mechanofluorescent polymer/silsesquioxane composites with high visibility and outstanding tunable mechanical properties were prepared by introducing tetraarylsuccinonitrile (TASN) derivatives, whose central carbon–carbon bonds can be cleaved upon exposure to mechanical stress, which generates relatively stable pink radicals and yellow-light emission under UV irradiation, via a sol–gel method. The mechanochromic and mechanical properties of the resulting materials were investigated using electron paramagnetic resonance (EPR) measurements and tensile tests, which revealed that these two properties are mutually related and can be easily tuned by changing the feed ratio of the starting materials.
Mechanochromic polymers change colour in response to mechanical stimuli. Such polymers have attracted great attention in materials science as they allow the detection of mechanical damage to materials, and thus enable the damaged materials to be replaced or repaired prior to the occurrence of serious fractures.1–22 The main strategy for the construction of mechanochromic polymers involves the incorporation of mechanoresponsive units (mechanochromophores) into the polymer chains. Several series of mechanochromophores have been reported so far. For example, Sottos et al. have reported pioneering work based on spiropyran mechanochromophores embedded into polymer chains, which exhibit a colour change from yellow to red/purple upon exposure to mechanical stress.23 Moreover, Sijbesma et al. have reported that sterically hindered dioxetane units incorporated into polymer chains or polymer networks emit visible chemiluminescence when subjected to mechanical force in the bulk,24 which enables increased detectability. Furthermore, systems with mechanofluorochromism, i.e., the activation or alteration of fluorescence via mechanical stress, have been introduced for persistent ultra-sensitive detection.15,25–31
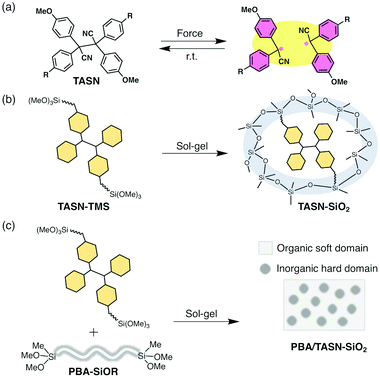
To achieve wide-spread applications of such mechanochromic polymers, high sensitivity to stress and tunable mechanical properties are desirable in order to minimize the amount of mechanochromophores that have to be incorporated into the polymeric materials. Recently, we have developed tetraaryl-succinonitrile (TASN) as a radical-type mechanochromophore that can be cleaved in response to mechanical stress and that generates the corresponding pink carbon-centred radicals that emit yellow light under UV irradiation (Fig. 1a).6 In addition to offering high visibility and sensitivity for stress detection, the cleavage of TASN can also be evaluated quantitatively using electron paramagnetic resonance (EPR) spectroscopy. Thus far, TASN has been introduced into polystyrenes and at the cross-linking points of polymer gels.6,12,13 If TASN could be successfully incorporated into polymer/silsesquioxane composites with tunable mechanical properties, it could bestow this class of materials with highly sensitive mechano-fluorescence.
We have previously demonstrated that the rigid framework of the hard silica domain maximizes the performance of radical-type mechanophores by moderately limiting their mobility, especially at the interface between hard and soft domains.7,32,33 In this paper, mechanofluorescent polymer/silsesquioxane composites with high visibility and outstanding tunable mechanical properties were prepared by introducing a TASN derivative via the sol–gel method.
A TASN derivative with trimethoxysilyl groups (TASN-TMS) was prepared and the sol–gel method was applied to afford rigid TASN-based networks (Fig. 1b). These TASN-based rigid networks were then embedded into cross-linked soft polymer elastomers via the sol–gel method to tune their mechanical properties for further applications and to reveal the correlation between their mechanochromic and mechanical properties (Fig. 1c).
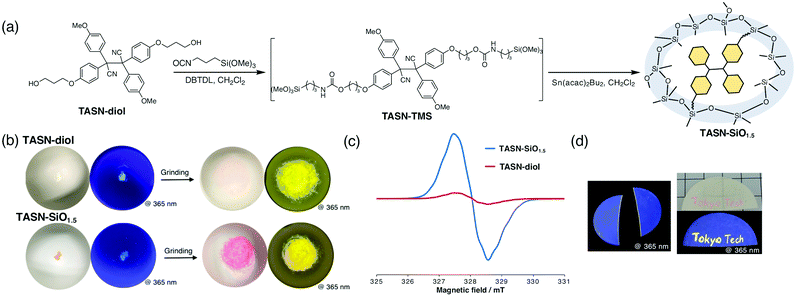
As shown in Fig. 2a, TASN-TMS was synthesized by reacting a TASN derivative that contains two hydroxy groups (TASN-diol) with 3-isocyanatopropyltrimethoxysilane (IPTMS) in the presence of a catalytic amount of di-n-butyltin dilaurate (DBTDL).34 The 1H NMR spectrum of the reaction mixture showed a new signal, which was assigned to a urethane linkage, and a shift of the methylene proton peaks relative to the TASN-diol and IPTMS spectra was observed (Fig. S1, ESI†). The integral ratios of these peaks were in good agreement with the IPTMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TASN-diol feed ratio. In addition, the gel permeation chromatography curve of the reaction mixture showed a unimodal distribution that was shifted to a higher molecular weight relative to the curve of TASN (Fig. S2 and S3, ESI†). These observations support the successful synthesis of TASN-TMS. To confirm the reactivity of TASN-TMS, dibutyltin bis(2,4-pentanedionate) (Sn(acac)2Bu2) was added to the TASN-TMS reaction mixture, which was not subjected to any purification in order to avoid hydrolysis of the methoxysilyl groups. The mixture was then cast in a Petri dish and cured for a week in air at room temperature to yield the TASN-based rigid silica networks as a yellow solid (TASN-SiO1.5).
TASN-diol feed ratio. In addition, the gel permeation chromatography curve of the reaction mixture showed a unimodal distribution that was shifted to a higher molecular weight relative to the curve of TASN (Fig. S2 and S3, ESI†). These observations support the successful synthesis of TASN-TMS. To confirm the reactivity of TASN-TMS, dibutyltin bis(2,4-pentanedionate) (Sn(acac)2Bu2) was added to the TASN-TMS reaction mixture, which was not subjected to any purification in order to avoid hydrolysis of the methoxysilyl groups. The mixture was then cast in a Petri dish and cured for a week in air at room temperature to yield the TASN-based rigid silica networks as a yellow solid (TASN-SiO1.5).
The mechanical activation of the TASN units in the rigid silica networks (TASN-SiO1.5) was demonstrated by grinding TASN-SiO1.5 with a pestle in a mortar. The obtained yellow solid rapidly turned pink and exhibited yellow emission under UV irradiation. The intensity of the pink colour and the fluorescence were clearly improved in comparison to TASN-diol (Fig. 2b). EPR measurements were performed to further characterize this phenomenon. TASN-SiO1.5 and TASN-diol were ground using a milling machine under the same conditions, and their EPR spectra were measured. The g value (2.003) of the peak observed indicated that it originated from dissociated TASN radicals (Fig. 2c). The dissociation ratios of the TASN units in TASN-SiO1.5 and TASN-diol were calculated using 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) as a standard. The dissociation ratio of the TASN units in TASN-SiO1.5 (0.14%) was 19 times higher than that of TASN-diol (0.0075%). The increase in the dissociation ratio should be attributed predominantly to the rigid covalent network of TASN-SiO1.5, which effectively transferred the mechanical force to the incorporated TASN mechanophores without energy loss due to molecular motion. The ground TASN-SiO1.5 sample retained the pink colour and yellow emission under UV irradiation for more than a week in air. The ratio of dissociated![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intact TASN units reached an equilibrium under the applied grinding conditions for TASN-SiO1.5. As the recombination of radicals strongly depends on their mobility, the slow recombination process in TASN-SiO1.5 suggests that the cross-linked silica networks restrict their mobility (Fig. S4, ESI†).
intact TASN units reached an equilibrium under the applied grinding conditions for TASN-SiO1.5. As the recombination of radicals strongly depends on their mobility, the slow recombination process in TASN-SiO1.5 suggests that the cross-linked silica networks restrict their mobility (Fig. S4, ESI†).
To further expand the applicability of TASN-TMS, a second composite material was prepared. Filter paper was immersed in TASN-TMS solution in CH2Cl2, which underwent a spontaneous sol–gel reaction. The obtained paper retained its pliability and exhibited a colour change and yellow fluorescence under UV irradiation upon being scratched or cut (Fig. 2d). This result indicates that the addition of a small amount of TASN-TMS can be used to endow various materials with mechanochromic properties.
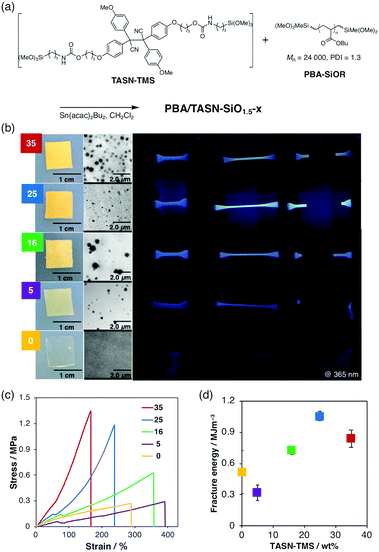
Although TASN-SiO1.5 exhibited a remarkable mechanoresponsiveness, the material was brittle. Taking advantage of the ability of the sol–gel method to incorporate a flexible and mechanically tough polymer into a soft domain, we synthesized mechanochromic elastomers (Fig. 3a). For that purpose, a series of polymer/silsesquioxane composite elastomers with different contents of TASN-TMS, PBA/TASN-SiO1.5-x (where x indicates the weight content (wt%) of TASN-TMS), was prepared via a sol–gel reaction between TASN-TMS and poly(butyl acrylate) (PBA) with dimethoxymethylsilyl groups at both ends (PBA-SiOR).
The obtained elastomers PBA/TASN-SiO1.5-x (x = 0, 5, 16, 25 and 35) were flexible. Transmission electron microscopy (TEM) images demonstrated that the sphere-like silica domains in the PBA matrix increased with increasing weight percentage of the TASN-TMS in the feed (Fig. 3b). To investigate the mechanical properties of PBA/TASN-SiO1.5-x, dumbbell-shaped films were prepared and subjected to tensile tests. The stress–strain curves of the PBA/TASN-SiO1.5-x samples (x = 16, 25 and 35) that exhibited fluorescence under UV irradiation when stretched (Fig. 3b) demonstrated that a higher content of TASN-TMS resulted in increased toughness, i.e., higher moduli and higher fracture energies (Fig. 3c). Thus, the mechanical properties of the obtained elastomers could be tuned by changing the feed ratio. In terms of mechano-fluorescence, PBA/TASN-SiO1.5-25 showed the best performance. This is probably due to its high stretchability, which might lead to the activation of TASN moieties at the interface between the soft and hard domains during the stretching process.7 We performed EPR measurement of the elastomer PBA/TASN-SiO1.5-25 during stretching. As a result, we confirmed that a very small amount of radicals (dissociation ratio of TASN is almost 0.0005%) were generated in the elastomer system during the stretching process (Fig. S6, ESI†), which is much lower than that of TASN-SiO1.5 (0.14%) by grinding. Although it was quite difficult to conclude that the dissociation ratio tended to be maximized immediately before rupture from the result of EPR measurement in which the radical amount is extremely small, remarkable fluorescence performance, high visibility and sensitivity for stress detection originating from the dissociated TASN can visually support the increase of generated radicals just before rupture as shown in Movie S1 (ESI†).
In conclusion, we have established a method for the synthesis of mechanochromic materials with high sensitivity and outstanding tunable mechanical properties by combining TASN derivatives and the sol–gel reaction. TASN derivatives functionalized with trimethoxysilyl groups (TASN-TMS) successfully form inorganic silica domains by the sol–gel reaction and act as mechanophores with high visibility and sensitivity in the composites. The rigid networks prepared using only TASN-TMS (TASN-SiO1.5) show high mechano-responsiveness and are able to endow ordinary paper with mechanochromic properties via an operationally simple procedure. A sol–gel reaction between TASN-TMS and a soft poly(butyl acrylate) polymer capped with dimethoxy methyl silyl groups produced a series of organic–inorganic composite elastomers (PBA/TASN-SiO1.5-x; x = 0, 5, 16, 25 and 35) that showed different mechanochromic and mechanical properties. These results, in conjunction with a quantitative EPR analysis, clearly demonstrated the utility of combining TASN-TMS and the sol–gel method for the preparation of materials with high mechanochromic visibility, which originates from the light-emitting TASN skeleton.
Conflicts of interest
There are no potential conflicts of interest to declare.Acknowledgements
This work was supported by KAKENHI grant 17H01205 (H. O.) from the Japan Society for the Promotion of Science (JSPS).Notes and references
- H. Zhang, X. Li, Y. Lin, F. Gao, Z. Tang, P. Su, W. Zhang, Y. Xu, W. Weng and R. Boulatov, Multi-modal mechanophores based on cinnamate dimers, Nat. Commun., 2017, 8, 1147 CrossRef PubMed.
- K. Imato, T. Kanehara, T. Ohishi, M. Nishihara, H. Yajima, M. Ito, A. Takahara and H. Otsuka, Mechanochromic Dynamic Covalent Elastomers: Quantitative Stress Evaluation and Autonomous Recovery, ACS Macro Lett., 2015, 4, 1307–1311 CrossRef CAS.
- B. R. Crenshaw and C. Weder, Deformation-Induced Color Changes in Melt-Processed Photoluminescent Polymer Blends, Chem. Mater., 2003, 15, 4717–4724 CrossRef CAS.
- Y. Chen and R. P. Sijbesma, Dioxetanes as mechanoluminescent probes in thermoplastic elastomers, Macromolecules, 2014, 47, 3797–3805 CrossRef CAS.
- A. D. N. Celestine, B. A. Beiermann, P. A. May, J. S. Moore, N. R. Sottos and S. R. White, Fracture-induced activation in mechanophore-linked, rubber toughened PMMA, Polymer, 2014, 55, 4164–4171 CrossRef CAS.
- T. Sumi, R. Goseki and H. Otsuka, Tetraarylsuccinonitriles as mechanochromophores to generate highly stable luminescent carbon-centered radicals, Chem. Commun., 2017, 53, 11885–11888 RSC.
- T. Kosuge, K. Imato, R. Goseki and H. Otsuka, Polymer-Inorganic Composites with Dynamic Covalent Mechanochromophore, Macromolecules, 2016, 49, 5903–5911 CrossRef CAS.
- M. K. Beyer and H. Clausen-Schaumann, Mechanochemistry: the mechanical activation of covalent bonds, Chem. Rev., 2005, 105, 2921–2948 CrossRef CAS PubMed.
- G. I. Peterson, M. B. Larsen, M. A. Ganter, D. W. Storti and A. J. Boydston, 3D-printed mechanochromic materials, ACS Appl. Mater. Interfaces, 2015, 7, 577–583 CrossRef CAS PubMed.
- K. Imato, J. C. Natterodt, J. Sapkota, R. Goseki, C. Weder, A. Takahara and H. Otsuka, Dynamic covalent diarylbibenzofuranone-modified nanocellulose: mechanochromic behaviour and application in self-healing polymer composites, Polym. Chem., 2017, 8, 2115 RSC.
- G. R. Gossweiler, G. B. Hewage, G. Soriano, Q. Wang, G. W. Welshofer, X. Zhao and S. L. Craig, Mechanochemical activation of covalent bonds in polymers with full and repeatable macroscopic shape recovery, ACS Macro Lett., 2014, 3, 216–219 CrossRef CAS.
- S. Kato, K. Ishizuki, D. Aoki, R. Goseki and H. Otsuka, Freezing-Induced Mechanoluminescence of Polymer Gels, ACS Macro Lett., 2018, 7, 1087–1091 CrossRef CAS.
- S. Kato, D. Aoki and H. Otsuka, Introducing static cross-linking points into dynamic covalent polymer gels that display freezing-induced mechanofluorescence: Enhanced force transmission efficiency and stability, Polym. Chem., 2019, 10, 2636–2640 RSC.
- M. M. Caruso, D. A. Davis, Q. Shen, S. A. Odom, N. R. Sottos, S. R. White and J. S. Moore, ChemInform Abstract: Mechanically-Induced Chemical Changes in Polymeric Materials, ChemInform, 2010, 41, 5755–5798 Search PubMed.
- Y. Chen, H. Zhang, X. Fang, Y. Lin, Y. Xu and W. Weng, Mechanical activation of mechanophore enhanced by strong hydrogen bonding interactions, ACS Macro Lett., 2014, 3, 141–145 CrossRef CAS.
- F. Verstraeten, R. Göstl and R. P. Sijbesma, Stress-induced colouration and crosslinking of polymeric materials by mechanochemical formation of triphenylimidazolyl radicals, Chem. Commun., 2016, 52, 8608–8611 RSC.
- T. Kobashi, D. Sakamaki and S. Seki, N-Substituted Dicyanomethylphenyl Radicals: Dynamic Covalent Properties and Formation of Stimuli-Responsive Cyclophanes by Self-Assembly, Angew. Chem., Int. Ed., 2016, 55, 8634–8638 CrossRef CAS PubMed.
- S. Y. Cho, J. G. Kim and C. M. Chung, A fluorescent crack sensor based on cyclobutane-containing crosslinked polymers of tricinnamates, Sens. Actuators, B, 2008, 134, 822–825 CrossRef CAS.
- A. L. Black, J. M. Lenhardt and S. L. Craig, From molecular mechanochemistry to stress-responsive materials, J. Mater. Chem., 2011, 21(6), 1655–1663 RSC.
- E. Ducrot, Y. Chen, M. Bulters, R. P. Sijbesma and C. Creton, Toughening Elastomers with Sacrificial Bonds and Watching Them Break, Science, 2014, 344(6180), 186–189 CrossRef CAS PubMed.
- M. J. Robb, T. Ann Kim, A. J. Halmes, S. R. White, N. R. Sottos and J. S. Moore, Regioisomer-Specific Mechanochromism of Naphthopyran in Polymeric Materials Scheme 1. Transformation of Naphthopyran into a Colored Merocyanine Species Is Accomplished Using Mechanical Force a, J. Am. Chem. Soc., 2016, 138(38), 12328–12331 CrossRef CAS PubMed.
- Z. Chen, J. A. M. Mercer, X. Zhu, J. A. H. Romaniuk, R. Pfattner, L. Cegelski, T. J. Martinez, N. Z. Burns and Y. Xia, Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene, Science, 2017, 357(6350), 475–479 CrossRef CAS PubMed.
- D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore and N. R. Sottos, Force-induced activation of covalent bonds in mechanoresponsive polymeric materials, Nature, 2009, 459, 68–72 CrossRef CAS PubMed.
- Y. Chen, A. J. H. Spiering, S. Karthikeyan, G. W. M. Peters, E. W. Meijer and R. P. Sijbesma, Mechanically induced chemiluminescence from polymers incorporating a 1,2-dioxetane unit in the main chain, Nat. Chem., 2012, 4, 559–562 CrossRef CAS PubMed.
- R. Göstl and R. P. Sijbesma, π-Extended Anthracenes As Sensitive Probes for Mechanical Stress, Chem. Sci., 2016, 7, 370–375 RSC.
- J. W. Kim, Y. Jung, G. W. Coates and M. N. Silberstein, Mechanoactivation of Spiropyran Covalently Linked PMMA: Effect of Temperature, Strain Rate, and Deformation Mode, Macromolecules, 2015, 48, 1335–1342 CrossRef CAS.
- J. R. Hemmer, P. D. Smith, M. Van Horn, S. Alnemrat, B. P. Mason, J. Read De Alaniz, S. Osswald and J. P. Hooper, High Strain-Rate Response of Spiropyran Mechanophores in PMMA, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 1347–1356 CrossRef CAS.
- C. K. Lee, B. A. Beiermann, M. N. Silberstein, J. Wang, J. S. Moore, N. R. Sottos and P. V. Braun, Exploiting force sensitive spiropyrans as molecular level probes, Macromolecules, 2013, 46, 3746–3752 CrossRef CAS.
- C. M. Degen, P. A. May, J. S. Moore, S. R. White and N. R. Sottos, Time-Dependent Mechanochemical Response of SP-Cross-Linked PMMA, Macromolecules, 2013, 46, 8917–8921 CrossRef CAS.
- C. M. Kingsbury, P. A. May, D. A. Davis, S. R. White, J. S. Moore and N. R. Sottos, Shear activation of mechanophore-crosslinked polymers, J. Mater. Chem., 2011, 21, 8381–8388 RSC.
- B. A. Beiermann, D. A. Davis, S. L. B. Kramer, J. S. Moore, N. R. Sottos and S. R. White, Environmental effects on mechanochemical activation of spiropyran in linear PMMA, J. Mater. Chem., 2011, 21, 8443–8447 RSC.
- T. Kosuge, X. Zhu, V. M. Lau, D. Aoki, T. J. Martinez, J. S. Moore and H. Otsuka, Multicolor Mechanochromism of a Polymer/Silica Composite with Dual Distinct Mechanophores, J. Am. Chem. Soc., 2019, 141, 1898–1902 CrossRef CAS PubMed.
- T. Kosuge, D. Aoki and H. Otsuka, Network reorganization in cross-linked polymer/silica composites based on exchangeable dynamic covalent carbon–carbon bonds, Polymer, 2019, 177, 10–18 CrossRef CAS.
- R. Yoneyama, T. Sato, K. Imato, T. Kosuge, T. Ohishi, Y. Higaki, A. Takahara and H. Otsuka, Autonomously Substitutable Organosilane Thin Films Based on Dynamic Covalent Diarylbibenzofuranone Units, Chem. Lett., 2016, 45, 36–38 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, instrumentation, synthesis and spectroscopic data. See DOI: 10.1039/c9qm00535h |
| This journal is © the Partner Organisations 2019 |