Tough biodegradable chitosan–gelatin hydrogels via in situ precipitation for potential cartilage tissue engineering†
Zhi-Sen Shen*abc,
Xiang Cuiac,
Rui-Xia Houc,
Qun Lia,
Hong-Xia Dengb and
Jun Fu *c
*c
aSchool of Medicine, Ningbo University, Ningbo 315211, China. E-mail: szs7216@163.com
bLi Huili Hospital Affiliated to Medicine of Ningbo University, Ningbo 315041, China
cCixi Institute of Biomedical Engineering, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Cixi, China. E-mail: fujun@nimte.ac.cn
First published on 18th June 2015
Abstract
Tough porous chitosan–gelatin (CG) hydrogels have been prepared through in situ precipitation with tunable porosity and degradation rates by adjusting the preparation formulations. Compression tests showed a Young's modulus of 3.25 MPa and a compressive strength of 2.15 MPa for the optimized C4G4 hydrogels, which are similar to or higher than those for human cartilage. Cyclic compression tests showed overlapping hysteresis loops for several cycles, with a compressive toughness of about 75.8 J m−2. In vitro enzymatic degradation results showed a degradation of 65.9% in 70 days, comparable to the regeneration rate of engineered cartilage reported in literature. Moreover, in vitro cell culture experiments showed excellent adhesion and proliferation of human thyroid cartilage cells on these hydrogels, where the chondrocytes function to secrete ECM. These tough and biodegradable hydrogels may have potential applications for cartilage tissue engineering.
1 Introduction
Damaged human cartilage caused by cancer, stenosis or trauma is hard to heal and prone to degeneration1 due to its lack of nervous and blood supply.2–5 Artificial substitutes and osteochondral allografts or autografts6 have been clinically employed to repair cartilage.7 However, the shortage of donor organs, the need of lifelong immunosuppression, and complications remain as major clinical obstacles.8 Alternative strategies are desired to repair and reconstruct cartilages.Recently, the rapid development of tissue engineering offers opportunities for repairing cartilage defects and restoring the function. Natural biomaterials have been used to fabricate constructs mimicking the extracellular matrix (ECM), in combination with a concert of biomolecules in order to regulate the growth, differentiation and/or proliferation of cells. As a load-bearing tissue, articular cartilages withstand compressive stress of several MPa. However, natural polymer hydrogel-based scaffolds are usually too soft and weak.9 It is desired to develop hydrogels with high strength for potential applications to load-bearing tissue regeneration.
Chitosan has been widely investigated for biomedical use including drug delivery, DNA vehicle, nerve reconstruction, and cartilage tissue engineering, etc.10–14 It possesses structural similarities to glycosaminoglycans (GAG), which are important structural components of cartilage ECM and play a pivotal role in modulating chondrocyte morphology, differentiation and function.15,16 Moreover, chitosan can be easily molded into porous structures to favor osteoconduction and minimize local inflammation.17 The abundant functional groups allow for chemical modification and crosslinking. Nevertheless, for applications as scaffolds for load-bearing tissues, chitosan-based materials are usually limited by their poor mechanical strength and elasticity.18,19
Gelatin is derived from collagen, a crucial component of cartilage ECM, and favorable to cell attachment.20 Gelatin has relatively low antigenicity because it's denatured.21 Moreover, gelatin is able to uptake high water content, which is favorable for nutrient transport and exchange in scaffolds. Recently, gelatin-based biomaterials have been applied to artificial skin, bone grafts, wound dressing materials and cartilage tissue engineering.22,23
Three-dimensional (3D) porous chitosan–gelatin (CG) scaffolds24 show excellent hydrophilicity and biodegradability, but low mechanical properties for cartilage tissue engineering. CG scaffolds fabricated by lyophilization25 showed a compression modulus of 47.9–72.5 kPa, which was 9–12 times higher than pure chitosan scaffolds. CG scaffolds obtained by a combined freeze-drying and glutaraldehyde cross-linking showed a compressive strength up to 264 ± 10.1 KPa,26 which is much lower than that for cartilages (e.g., 8.4–15.5 MPa for articular cartilage,27 and 0.8 MPa for soft human nasal septum cartilage28). It is challenging to further improve the mechanical strength and toughness of CG scaffolds for cartilage regeneration.
Herein, this paper used an in situ precipitation method29,30 to fabricate CG hydrogels with improved mechanical properties, as well as biodegradability and biocompatibility. Compression tests have been conducted on hydrogels with different formulations. The fatigue resistance and toughness of the hydrogels were systematically investigated by performing cyclic compression tests. In vitro degradation experiments demonstrated that the hydrogels were biodegradable with adjustable degradation rates. Hydrogels are shown non-toxic and supportive to the adhesion and proliferation of chondrocytes.
2 Materials and methods
2.1 Materials
Medical grade chitosan (CS, with a viscosity-average molecular weight (Mη) of 106, and the degree of N-deacetylation (DD) of 95%) was supplied by Aladdin Chemical Reagent Co., Ltd., China. Gelatin (MW: ∼60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), acetic acid and NaOH were analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd., China. Lysozyme from chicken egg white (≥5000 units per mg dry weight) was supplied by Aladdin Chemical Reagent Co., Ltd., China.
000), acetic acid and NaOH were analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd., China. Lysozyme from chicken egg white (≥5000 units per mg dry weight) was supplied by Aladdin Chemical Reagent Co., Ltd., China.
2.2 Preparation of chitosan–gelatin (CG) scaffolds
CG hydrogels were prepared by the following procedure. First, chitosan and gelatin powders were dissolved in 2% (v/v) acetic acid solution with stirring at 50 °C in water bath for 24 h before being placed stationary to remove air bubbles (Fig. 1a). On the other hand, viscous chitosan solution (5% m/v, about 5 mL) was cast uniformly into a cylindrical mould to create a thin layer of solution on the wall of the mould. The liquid layer was precipitated by soaking in 5% (w/w) NaOH solution for 10 min, leading to the formation of chitosan membrane on the internal surface of the mould (Fig. 1b). Then the mould was removed from the NaOH solution. Subsequently, the CG solution was poured into the mould before being immersed into 5% (w/w) NaOH again. After in situ precipitation for 12 h, a rod-like CG gel was constructed. The obtained rod was rinsed with distilled water to neutral pH and then dried in an oven at 55 °C for specific durations (e.g., 0, 1, 2, 3, and 4 h). The dried gels were frozen at −70 °C for 24 h and lyophilized for 48 h to obtain porous cylindrical CG scaffolds with 10 mm length and 15 mm diameter. The mass ratio of chitosan to gelatin was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 4
0, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 4
2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 4
4, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (with each designated as C4G0, C4G2, C4G4, and C4G8).
8 (with each designated as C4G0, C4G2, C4G4, and C4G8).
2.3 Measurement of hydrogel porosity
The porosity of hydrogels was measured by using a liquid displacement method. Ethanol was used as it is easy to permeate into the interior of materials without inducing shrinking or swelling. Typically, a dried hydrogel sample was immersed in ethanol with a volume V1, with air removed by cyclic evacuation. The total volume of the sample and ethanol was marked as V2. Thus, the occupied volume of scaffolds was calculated as V2 − V1. Subsequently, the ethanol-containing sample was removed, leaving ethanol with a volume V3. Then the ethanol volume inside the sample was V1 − V3. Therefore, the sample volume V is a sum of the occupied volume and the pore volume. That is, V = (V2 − V1) + (V1 − V3) = V2 − V3. So the porosity of chitosan scaffolds is calculated as P = (V1 − V3)/(V2 − V3).2.4 Swelling property
The swelling properties of the chitosan–gelatin scaffolds were investigated. Briefly, the scaffolds were immersed in phosphate buffer saline (PBS, pH = 7.4) at room temperature for 90 min. The samples were retrieved in a period of predetermined time and the excess water was removed using a filter paper. The wet weight of the scaffold (Ws) was determined using an electronic balance, after which the swollen scaffold was dried in an oven at 50 °C and the dry weight was Wd. Three samples each were used to make an average. The swelling ratio of scaffolds was calculated as: S = (Ws − Wd)/Wd.2.5 Compression tests
Cylindrical specimens (9–11 mm height and 15 mm diameter, n = 3 each) with different drying times and formulations were saturated with 0.1 M PBS water and compressed at 10% min−1 by using an Instron 5567 machine (Instron Inc., MA) to predetermined strains. The slope of the stress–strain curve at 10–20% strain was used to calculate the elastic modulus (E). Moreover, the specimens were subjected to a series of cyclic compression tests to 20%, 40%, or 60% strains for five cycles. Before testing, all samples were swollen in water for 30 min.2.6 In vitro biodegradation
The biodegradation of chitosan–gelatin (CG) hydrogels was investigated in vitro by using lysozyme as catalyst. Typically, CG samples (3 mm × 3 mm × 3 mm, weight = 20 mg) were incubated with 13 mg L−1 lysozyme in 0.1 M PBS (pH = 7.4). In a control group, the same specimens were incubated in 0.1 M PBS without lysozyme. All the specimens were incubated at 37 °C for 10 weeks and solutions were refreshed once a week. The specimens were taken out from the medium, washed with distilled water, freeze-dried, and weighed. The degradation ratio D was calculated as D = (W0 − Wt)/W0 × 100%, where W0 denotes the original weight and Wt is the weight at time t. Three specimens were used for each hydrogel. The results were reported as mean ± standard deviation in the text.2.7 In vitro cell culture with scaffolds
Chondrocytes were isolated from thyroid cartilage of the total laryngectomy patients with an informed consent from the patient and the approval by the Ethics Committee of Lihuili Hospital, School of Medicine, Ningbo University. The obtained thyroid cartilage was cut into small pieces, which were incubated in D-Hanks containing 0.25% trypsin for (5 mL) for 40 min and 0.2% collagenase type II (5 mL) for 6 h. The isolated cells were seeded into cell culture flasks at a density of 105 cells per cm2 and cultivated in a humidified atmosphere of 5% CO2 at 37 °C. Cells were supplied with DMEM containing 20% fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The medium was replaced every other day. After around 85% cells overspread the culture flasks, cells were detached and re-suspended in DMEM for further experiments. Cells at passage 6 were used.Lyophilized C4G4 and C4G0 hydrogels (5 mm diameter and 1 mm height) were sterilized by incubation in 75% ethanol, followed by washing in sterile PBS. The chondrocytes (1.5 × 105 cells per mL) were seeded to hydrogels in 48-well plates (Corning) (n = 3). The cell-scaffold constructs were added with complete medium and cultured in a humidified incubator at 37 °C with 5% CO2. The culture medium was changed every other day until harvest. After 1 and 7 day culture, the cells adhered to scaffolds were fixed with 2.5% glutaraldehyde at 4 °C for 24 h. After thorough washing with 0.1 M PBS (pH 7.4), the hydrogels were dehydrated in ethanol in a sequential manner (40%, 60%, 80% and 100%) for 10 min each and allowed dry on a clean plate at room temperature.
2.8 Scanning electron microscopy (SEM)
CG hydrogels with different formulations, and cell-seeded hydrogels after in vitro culture were sputter coated with a thin gold layer for imaging by using a scanning electron microscope (SEM, TM-1000, Hitachi Japan) at 4 kV. The average pore size and wall thickness were estimated by using at least 30 pores of the hydrogels.2.9 Confocal laser scanning microscopy (CLSM)
The cell-seeded hydrogel samples cultured for 1, 3 and 7 days were washed three times with PBS and fixed with 2% glutaraldehyde for 12 h. The C4G4 specimens were washed with PBS and then soaked in 0.1% Triton X-100 for 10 min, washed again in PBS. The chondrocytes were stained with 50 μg mL−1 phalloidin-FITC (Invitrogen) solution for 1 h at room temperature, followed by multiple washing with PBS. Subsequently, 10 μg mL−1 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) was added and incubated in dark at room temperature for 5 min, and then the samples were washed with PBS. Finally, the cytoskeleton and nucleus of chondrocytes were imaged by using a CLSM (Leica TCS SP5 II, Braunschweig, Germany).2.10 CCK-8 assay
The cell growth and proliferation on the C4G4 and C4G0 hydrogels were assessed by using CCK-8 assay after incubation for 1, 3 or 5 days. At predetermined time, 100 μL CCK-8 solution was added to the incubated chondrocyte-seeded hydrogels in the medium. The mixtures were further incubated for 4 h before 100 μL solution of each well was sampled to determine the absorbance at 450 nm by using a microplate reader. The average of five parallel measurements was calculated for each group.3 Results and discussion
3.1 Structures and porosity of the chitosan–gelatin hydrogels
The in situ precipitation method was successfully used to prepare natural CG hydrogels. During the gelation process, the positively charged CS chains were precipitated due to the deprotonation of amino groups in the alkaline environment (Fig. 1). In this work, all the hydrogels showed three dimensional porous structures with orientation and controllable pore size. Fig. 2a shows the cross section SEM images along with the c axis of the C4G0 hydrogel. The pores exhibited a micropipe appearance with oriented structures. Meanwhile, the cross section perpendicular to the c axis shows typical interconnecting porous structures. The micropores were connected with each other (Fig. 2b). The high interconnectivity is essential to nutrient transportation and removal of metabolic wastes.Moreover, the pore diameter and wall thickness could be adjusted by changing the gelatin concentration and drying time. Chitosan hydrogels without gelatin showed pores with average diameters of 180–300 μm and wall thickness about 20 μm (Fig. 2a). In contrast, the C4G4 hydrogels with 4 wt% gelatin showed pores with an average diameter of about 100 μm and wall thickness about 20 μm (Fig. 2c). With a higher magnification, the C4G4 hydrogel showed a rough surface with many small gelatin particles, which may favor cell adhesion (Fig. 2d). In C4G8, due to the high gelatin concentration, the surface appeared very rough and disordered (ESI†).
Besides, the porosity plays a critical role in cell attachment, proliferation, and matrix deposition in hydrogels. For 1 h drying, the C4G0 hydrogel had a porosity of 76.9 ± 4.2%. In contrast, the hydrogel containing 4 wt% gelatin (C4G4) showed a porosity of 70.4 ± 2.1%. When the gelatin concentration was increased to 8%, the porosity was further decreased to 52.7 ± 5.9% (Fig. 3a). The decrease in porosity with increasing gelatin concentration is consistent with the SEM observation. On the other hand, the drying time had a significant influence on the porosity. For the C4G4 hydrogels, the porosity was decreased from 87.0 ± 4.2% for the as prepared hydrogel to 68.5 ± 2.8% after 1 h drying (Fig. 3b). This reduction in porosity is attributed to the water loss during drying.
 | ||
| Fig. 3 (a) The porosities of CG hydrogels prepared with different formulations. (b) The porosities of C4G4 hydrogels prepared with different drying time. | ||
Although there is a lack of consensus regarding the optimal pore size inside a porous polymer scaffold for cell growth and mass transport, cartilage scaffolds are generally suggested to have highly interconnected pores with a porosity higher than 70% and pore sizes between around 100 and 300 μm in order to facilitate cell ingrowth.31 In contrast to C4G8, both the C4G0 and C4G4 showed porous structures within this range. As the mechanical strength decreases with the increasing porosity, however, the void volume in scaffolds should be balanced to favor chondrocyte accommodation and to provide adequate strength and support. So an ideal scaffold should be a compromise between the mechanical stability and the interconnectivity of its porous network.
3.2 Swelling property
The swelling property is related to the crosslink density and important for the cell adhesion and growth. Fig. 4 compares the swelling ratios of C4G0, C4G4, and C4G8 scaffolds. With increasing gelatin content, the swelling ratio was decreased from 511.4% for C4G0 to 438.3% for C4G4 and 302.2% of C4G8 after 90 min swelling in PBS. These results suggest a denser network with increasing gelatin content, which is consistent with the microporous structures and porosity of the chitosan–gelatin hydrogels reported in Fig. 2 and 3. | ||
| Fig. 4 Swelling studies of hydrogels with different gelatin concentration in PBS (pH 7.4). Each point represents the mean of the results from three different specimen. | ||
3.3 Compressive properties of the hydrogels
The results shown above have suggested critical roles played by the drying time and formulation on the porous structures. Here, we further demonstrate that these factors significantly influence the mechanical properties of CG hydrogels.Compression tests showed very high strength and modulus of C4G4 gels beyond those for the pure chitosan gels (Table 1). The fracture strain (εf) of C4G4 gels was as high as 70.04 ± 1.27% and the fracture strength (σf) was up to 2.2 ± 0.54 MPa, in contrast to εf of 68.24 ± 1.12% and σf of 0.76 ± 0.11 MPa for the C4G0 gels. Meanwhile, the corresponding Young's modulus of C4G4 gels was 3.2 ± 0.12 MPa, in contrast to 1.06 ± 0.06 MPa for the C4G0 gels. Furthermore, when the gelatin content was increased from 4% to 8%, the corresponding strength and modulus were further raised to 11.32 ± 1.42 MPa and 10.24 ± 1.05 MPa (Fig. 5a and b). These results suggest that gelatin plays a critical role in the enhancement of mechanical strength and toughness. It is likely that there are strong electrostatic interactions between chitosan and gelatin (Fig. 1b).
| Sample | Chitosan (wt%) | Gelatin (wt%) | Porosity (%) | Compressive modulus (MPa) | Fracture stress (MPa) | Fracture strain (%) |
|---|---|---|---|---|---|---|
| C4G0 | 4 | 0 | 76.9 ± 4.2 | 1.06 ± 0.06 | 0.76 ± 0.11 | 68.24 ± 1.12 |
| C4G2 | 4 | 2 | 74.1 ± 3.9 | 0.73 ± 0.13 | 0.71 ± 0.07 | 64.83 ± 2.88 |
| C4G4 | 4 | 4 | 70.4 ± 2.1 | 3.2 ± 0.12 | 2.2 ± 0.54 | 70.04 ± 1.27 |
| C4G8 | 4 | 8 | 52.7 ± 5.9 | 10.2 ± 1.05 | 11.3 ± 1.42 | 87.8 ± 3.25 |
Besides, the drying time also has remarkable influence on the mechanical properties (Fig. 5c and d). The compressive strength and modulus were increased to 11.24 ± 0.44 MPa and 21.8 ± 1.58 MPa after 4 h drying, in contrast to the compressive strength of 0.53 ± 0.20 MPa and modulus of 0.46 ± 0.42 MPa for the 0 h drying gels. The increase in mechanical strength is largely attributed to the water loss during drying.
Furthermore, we compare the mechanical performance of CG hydrogels to those of human cartilages. The Young's modulus of C4G4 hydrogel (3.25 MPa) is higher than that for human cartilage. For example, the compressive modulus of human nasoseptal cartilage, articular cartilage and knee meniscus was reported as 0.8 MPa, 0.31–0.85 MPa and 0.17–0.35 MPa,32–34 which are lower than that of C4G4.
The fatigue resistance of C4G4 hydrogels was investigated by performing cyclic loading–unloading compressive tests at constant strains. Fig. 6 shows five immediate consecutive loading–unloading curves of C4G4 gel. The hysteresis loop represents the energy dissipation during loading–unloading test. With a maximum of 60% strain, the area of the hysteresis loops decayed with increasing loading cycles (Fig. 6a). Meanwhile, the stress at 60% strain (σ0.60) of C4G4 gels slightly decreases from 175 kPa for the first loading to 164, 157, 154 and 152 kPa for the second, third, fourth and fifth runs. With up to ten loading cycles, the C4G4 gels show σ0.60 about 150 kPa. On the other hand, five immediate consecutive loading–unloading tests were conducted on C4G4 hydrogels under maximum strains of 20%, 40% and 60%. The hysteresis energy, or the loop area, decreased from about 300.3 J m−2 for the first run to about 115.9 J m−2 for the second cycle at 60% strain (Fig. 6b). With more cycles, the loop area dropped to a plateau value of around 75 J m−2 (Fig. 6b). The same trend also exists for 20% and 40% strains. The plateau values suggests the outstanding fatigue resistance of hydrogels.
3.4 In vitro degradation
For cartilage tissue engineering, it is important that the porous scaffolds are biodegradable in a controlled manner. Lysozyme is known to catalyze the hydrolysis of peptidoglycan in chitosan35,36 and the degradation of gelatin. The enzymatic degradation of CG hydrogels for 70 days was compared with that in PBS (Fig. 7). In 70 days, the weight loss of C4G0 (L + P) was only 36.7 ± 2.55%, whereas that of C4G8 (L + P) was 88.7 ± 1.54%. The CG gel with a high gelatin content and low porosity showed a faster degradation. Herein, the degradation rate is dominated by the gelatin content, rather than the porosity. Furthermore, compared with CG (P), the degradation rate of CG (L + P) was improved from 31.5% to 36.7%, 42.8% to 45.9%, 59.8% to 65.9%, and 82.6% to 88.7% for C4G0 to C4G8 in 70 days. These results demonstrate the feasibility to control the degradation rate by manipulating the hydrogel formulation. | ||
| Fig. 7 Degradation of CG hydrogels with different formulations over time in PBS (solid symbols) or PBS with lysozyme (13 mg L−1, open symbols) (pH 7.4, T = 37 °C). | ||
For cartilage tissue engineering, it is pursued that the scaffold degradation rate matches the generation rate of new cartilage. Mason et al.37 had proved a complete articular cartilage regeneration at 12 weeks in a rabbit knee osteochondral defect model. Gelatin showed a very quick degradation of 63% at 72 h,38 while chitosan (C4G0) only degraded by 36.7% in 10 weeks. The degradation of chitosan is too slow while that of gelatin is too fast for cartilage engineering. Xia et al.39 fabricated CG hydrogels by lyophilization with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and implanted with auricular cartilage into subcutaneous tissue of a pig. After 16 weeks, the CG scaffolds were completely degraded and the harvested tissue showed mature and relatively homogeneous cartilage formation. Herein, the C4G4 was degraded by 65.9% in 10 weeks, which may match the regeneration rate of cartilage. Further in vivo degradation test should be done to test this hypothesis.
1 ratio and implanted with auricular cartilage into subcutaneous tissue of a pig. After 16 weeks, the CG scaffolds were completely degraded and the harvested tissue showed mature and relatively homogeneous cartilage formation. Herein, the C4G4 was degraded by 65.9% in 10 weeks, which may match the regeneration rate of cartilage. Further in vivo degradation test should be done to test this hypothesis.
3.5 Cell adhesion and proliferation
For applications in cartilage tissue engineering, cellular attachment and migration within scaffolds are essential for cartilage tissue adaptation. The scaffold should promote chondrocyte adhesion and allow for the retention of the metabolic functions of attached chondrocytes. In this section, human thyroid chondrocytes were seeded into and cultured in C4G4 and C4G0 hydrogels. According to SEM images, the chondrocytes were sparsely distributed on the surface of C4G4 and C4G0 gels on day 1 (Fig. 8a and b). The cells maintained their round shape on rough surface of C4G4, indicating a good cyto-affinity. In contrast, the cells adhered on the smooth surface of C4G0 appeared flat. After 7 day culture, more chondrocytes were observed on the surface of both C4G4 and C4G0 hydrogels (Fig. 8c and d). All the cells cling together and couldn't fully outstretch. The cells on C4G4 hydrogel maintained the original oval shape (Fig. 8c), but the cells cultivated on C4G0 remained flat.CLSM observations of chondrocytes stained with DAPI to nucleus (blue) and phalloidin-FITC to cytoskeleton (green) further show the adhesion and growth of cells on the C4G0 and C4G4 hydrogels (Fig. 9). The chondrocyte density on the C4G4 hydrogel is much higher than that on the C4G0 hydrogel after three-day culture. This is consistent with quantitatively characterization by CCK-8 assay (to be shown below).
The chondrocyte adhesion and growth on the C4G4 hydrogel for 1 and 7 days was imaged by using CLSM. The chondrocytes adhered uniformly on the hydrogel at day 1. In 7 days, the chondrocyte density was increased and spread uniformly on the hydrogel surface. Besides, the CLSM inspection on the cross-section of the C4G4 scaffolds cultured for 7 days show excellent ingrowth of cells into the pores (Fig. 10). These results suggest that the interconnected porous structures not only facilitate the nutrition and waste transportation, but also allow for the ingrowth of cells, which is very important for the regeneration of new tissues.
We further used CCK-8 assay to quantitatively evaluate the adhesion and proliferation of chondrocytes seeded on C4G4 and C4G0 hydrogels after 1, 3 and 5 days (Fig. 11). The OD values at 450 nm of the samples show an increase over time, suggesting an increase in chondrocyte density, or cell growth. Meanwhile, the cell number density on C4G4 gel was higher than that on the C4G0 gel. It is noted that, the cell adhesion and growth on the C4G0 and C4G4 hydrogels are much higher than those on the blank group with a standard culture plate as substrate. These results are consistent with the fluorescent observations and indicate that the chitosan-based hydrogels are excellent scaffolds for chondrocyte adhesion and growth.
Finally, we further demonstrate that the chondrocytes nicely maintain their phenotype by secreting extracellular matrix biomolecules. The C4G4 scaffolds cultured with chondrocytes for three days were stained with alcian blue staining, which selectively stains glycosaminoglycans in ECM. Abundant stained glycosaminoglycans were observed in blue surrounding the chondrocytes (Fig. 12). It suggests that the chondrocytes function to secrete glycosaminoglycan.
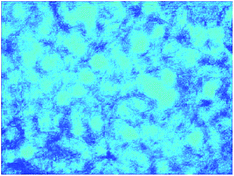 | ||
| Fig. 12 Optical micrograph of C4G4 hydrogel cultured with chondrocytes for three days. The scaffold was stained with alcian blue and imaged with a magnification of 400. | ||
4 Conclusions
In this paper, we prepared novel porous chitosan–gelatin scaffolds via an in situ precipitation method, resulting in strong, tough, and functional scaffolds. The optimal C4G4 scaffold has controlled biodegradability, cytocompatibility, microporous structures, and excellent mechanical properties. For example, the C4G4 hydrogel showed an average diameter of 100 μm and porosity of 68.5% ± 2.8, together with compressive strength and modulus of 2.15 MPa and 3.25 MPa respectively. These properties are close to human cartilages. Besides, in vitro enzymatic degradation experiments showed that the C4G4 hydrogel degraded 65.9% over 70 days, which may match the regeneration rate of cartilage as reported in literature. These scaffolds nicely support the human thyroid cartilage cells adhesion and growth, according to CLSM, CCK-8, and GAG staining. These novel porous scaffolds may have potential applications in cartilage tissue engineering.Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (no. LR13B040001, LY14H160003), Ningbo Innovative Team Program (no. 2012B82019), the Hundred Talents Program, Chinese Academy of Sciences (JF), Medical and Health Research Project of Zhejiang Province (no. 2012ZDA042), Medical and Health Training Project of Zhejiang Province (no. 2014PYA017), Ningbo Social Developmental Key Research Project (no. 2012C5015). Part of the experiments were conducted in the labs of Prof. Dr Jun-Ming Guo and Prof. Dr Ya-Bin Zhu.References
- G. Filardo, E. Kon, F. Perdisa, F. Balboni and M. Marcacci, Int. Orthop., 2014, 38, 1905–1912 CrossRef PubMed.
- R. S. Tuan, Arthritis Res. Ther., 2007, 9, 109 Search PubMed.
- J. Buckwalter and H. Mankin, Instr. Course Lect., 1997, 47, 487–504 Search PubMed.
- M. Pecina and S. Vukicevic, Int. Orthop., 2007, 31, 719–720 CrossRef PubMed.
- C. Vinatier, C. Bouffi, C. Merceron, J. Gordeladze, J.-M. Brondello, C. Jorgensen, P. Weiss, J. Guicheux and D. Noël, Curr. Stem Cell Res. Ther., 2009, 4, 318 CrossRef CAS.
- J. Farr, B. Cole, A. Dhawan, J. Kercher and S. Sherman, Clin. Orthop. Relat. Res., 2011, 469, 2696–2705 CrossRef PubMed.
- G. Musumeci, C. Loreto, S. Castorina, R. Imbesi, R. Leonardi and P. Castrogiovanni, Ital. J. Anat. Embryol., 2013, 118, 189–203 Search PubMed.
- D. Puppi, F. Chiellini, A. Piras and E. Chiellini, Prog. Polym. Sci., 2010, 35, 403–440 CrossRef CAS PubMed.
- H. Shin, B. D. Olsen and A. Khademhosseini, Biomaterials, 2012, 33, 3143–3152 CrossRef CAS PubMed.
- M. Dash, F. Chiellini, R. Ottenbrite and E. Chiellini, Prog. Polym. Sci., 2011, 36, 981–1014 CrossRef CAS PubMed.
- K. Dong, X. Guo, J. Xu, D. Yang and F. Qiu, Polym.-Plast. Technol. Eng., 2012, 51, 754–759 CrossRef CAS PubMed.
- A. Erdem, E. Eksin and M. Muti, Colloids Surf., B, 2014, 115, 205–211 CrossRef CAS PubMed.
- R. Muzzarelli and C. Muzzarelli, in Polysaccharides I, Springer, 2005, pp. 151–209 Search PubMed.
- G. Y. Zhu, Z. B. Xiao, R. J. Zhou and F. P. Yi, Adv. Mater. Res., 2012, 535, 440–445 CrossRef.
- W.-C. Chen, C.-L. Yao, I. Chu and Y.-H. Wei, J. Biosci. Bioeng., 2011, 111, 226–231 CrossRef CAS PubMed.
- M.-Y. Wu, N. Chen, L.-K. Liu, H. Yuan, Q.-L. Li and S.-H. Chen, J. Bioact. Compat. Polym., 2009, 24, 301–315 CrossRef CAS PubMed.
- H. Seeherman, R. Li and J. Wozney, J. Bone Jt. Surg., Am. Vol., 2003, 85, 96–108 Search PubMed.
- R. A. Muzzarelli, F. Greco, A. Busilacchi, V. Sollazzo and A. Gigante, Carbohydr. Polym., 2012, 89, 723–739 CrossRef CAS PubMed.
- S. Jana, S. J. Florczyk, M. Leung and M. Zhang, J. Mater. Chem., 2012, 22, 6291–6299 RSC.
- B. Young, W. Pitt and S. Cooper, J. Colloid Interface Sci., 1988, 124, 28–43 CrossRef CAS.
- A. O. Elzoghby, J. Controlled Release, 2013, 172, 1075–1091 CrossRef CAS PubMed.
- S.-M. Lien, W.-T. Li and T.-J. Huang, Mater. Sci. Eng., C, 2008, 28, 36–43 CrossRef CAS PubMed.
- X. Wu, Y. Liu, X. Li, P. Wen, Y. Zhang, Y. Long, X. Wang, Y. Guo, F. Xing and J. Gao, Acta Biomater., 2010, 6, 1167–1177 CrossRef CAS PubMed.
- S. C. Miranda, G. A. Silva, R. C. Hell, M. D. Martins, J. B. Alves and A. M. Goes, Arch. Oral Biol., 2011, 56, 1–15 CrossRef CAS PubMed.
- W. W. Thein-Han, J. Saikhun, C. Pholpramoo, R. D. K. Misra and Y. Kitiyanant, Acta Biomater., 2009, 5, 3453–3466 CrossRef CAS PubMed.
- J. K. He, D. C. Li, Y. X. Liu, H. X. Zhan, Q. Lian, B. H. Lu and Y. Lv, Acta Biomater., 2009, 5, 453–461 CrossRef CAS PubMed.
- W. W. Thein-Han and Y. Kitiyanant, J. Biomed. Mater. Res., Part B, 2007, 80, 92–101 CrossRef PubMed.
- A. Zemek, R. Garg and B. J. Wong, Laryngoscope, 2010, 120, 1089–1093 Search PubMed.
- Q. Hu, F. Chen, B. Li and J. Shen, Mater. Lett., 2006, 60, 368–370 CrossRef CAS PubMed.
- Q. Hu, B. Li, M. Wang and J. Shen, Biomaterials, 2004, 25, 779–785 CrossRef CAS.
- Y. Zhu, Y. Wan, J. Zhang, D. Yin and W. Cheng, Colloids Surf., B, 2014, 113, 352–360 CrossRef CAS PubMed.
- M. I. Baker, S. P. Walsh, Z. Schwartz and B. D. Boyan, J. Biomed. Mater. Res., Part B, 2012, 100, 1451–1457 CrossRef PubMed.
- C. P. Neu, T. Novak, K. F. Gilliland, P. Marshall and S. Calve, Osteoarthritis Cartilage, 2014, 23, 405–413 CrossRef PubMed.
- M. Sweigart and K. Athanasiou, Proc. Inst. Mech. Eng., Part H, 2005, 219, 53–62 CrossRef CAS.
- P. Zhao, T. Han, J. J. Guo, S. L. Zhu, J. Wang, F. Ao, M. Z. Jing, Y. L. She, Z. H. Wu and L. B. Ye, Virus Res., 2012, 169, 1–7 CrossRef CAS PubMed.
- M. Tan, H. Wang, Y. Wang, G. Chen, L. Yuan and H. Chen, J. Mater. Chem. B, 2014, 2, 569–576 RSC.
- J. M. Mason, A. S. Breitbart, M. Barcia, D. Porti, R. G. Pergolizzi and D. A. Grande, Clin. Orthop. Relat. Res., 2000, 379, S171–S178 CrossRef PubMed.
- C. R. Correia, L. S. Moreira-Teixeira, L. Moroni, R. L. Reis, C. A. van Blitterswijk, M. Karperien and J. F. Mano, Tissue Eng., Part C, 2011, 17, 717–730 CrossRef CAS PubMed.
- W. Xia, W. Liu, L. Cui, Y. Liu, W. Zhong, D. Liu, J. Wu, K. Chua and Y. Cao, J. Biomed. Mater. Res., Part B, 2004, 71, 373–380 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06835e |
| This journal is © The Royal Society of Chemistry 2015 |








