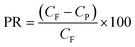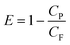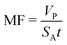Chemical treatment technologies for waste-water recycling—an overview
Vinod Kumar
Gupta
*ab,
Imran
Ali
c,
Tawfik A.
Saleh
b,
Arunima
Nayak
a and
Shilpi
Agarwal
c
aDepartment of Chemistry, Indian Institute of Technology Roorkee, Roorkee, 247 667, India. E-mail: vinodfcy@gmail.com; vinodfcy@iitr.ernet.in; Fax: 0091-1332-286202; Tel: 0091-1332-285801
bKing Fahd University of Petroleum and Minerals, Dhahran, 31261, Saudi Arabia
cChemistry Department, Jammia Millia Islamia, New Delhi India
First published on 4th April 2012
Abstract
The global population is increasing and because of this, the world may experience great fresh water scarcity. Our water resources are limited and, hence, water treatment and recycling methods are the only alternatives for getting fresh water in the coming decades. Therefore, there is a great need for the development of a suitable, inexpensive and rapid wastewater treatment techniques and reuse or conservation methods in the present century. The different types of water treatment and recycling techniques have been discussed in terms of their basic principles, applications, costs, maintenance and suitability. Additionally, a systematic approach to water treatment and recycling involving their understanding, evaluation and selection parameters has been presented. A brief guideline for the selection of the appropriate technologies for specific applications has been evaluated. This review adds to the global discussions on water scarcity solutions.
1. Introduction
Water, is of utmost importance in our daily lives, hence, the need to improve and preserve its quality is growing continuously. Point and non-point sources are contaminating our valuable water resources. The main water pollution sources are from industrial, domestic and agricultural activities and other environmental and global changes. The surface and ground water in many places around the world is contaminated and not fit for drinking purposes. By 2020, the global population is supposed to reach up to 7.9 billion1 and because of this the world may experience the great scarcity of fresh water.2,3In view of this, attempts have been made to compare various water treatment and recycling technologies. Efforts have also been carried out to introduce an approach for water treatment and recycling methods. A comparison of the technologies has been presented by discussing their performance, sludge production, life period and operation. The purpose of this article is to provide guidelines for the selection of the technologies or their combinations for various applications so that one can select the exact and correct technology.
2. Water pollutants
Prior to discussing water treatment and reclamation, one should be aware about the qualitative and quantitative nature of water pollutants. Many pollutants are present in wastewater but toxicity is only observed beyond a certain limit called the permissible limit. The type of pollutants present in the wastewater depends upon the nature of the industrial, agricultural and municipal wastewater releasing activities. The different types of water pollutants may be categorized as inorganic, organic, and biological in nature. The most common inorganic water pollutants are heavy metals, which are highly toxic and carcinogenic in nature. Additionally, nitrates, sulphates, phosphates, fluorides, chlorides and oxalates also have some serious hazardous effects. The toxic organic pollutants are from pesticides which includes insecticides, herbicides, fungicides; polynuclear hydrocarbons (PAHs), phenols, polychlorinated biphenyls, halogenated aromatic hydrocarbons, formaldehyde, polybrominated biphenyls, biphenyls, detergents, oils, greases etc. In addition to these, normal hydrocarbons, alcohols, aldehydes, ketones, proteins, lignin, pharmaceuticals etc. are also found in wastewater. Different types of microbes thriving in wastewater may be responsible for different type of diseases. The harmful microbes include bacteria, fungi, algae, plankton, amoeba, viruses and other worms. These water pollutants remain either in solvated, colloidal or in suspended form.4–63. Wastewater treatment and recycling technologies
Wastewater treatment and reuse is an important issue and scientists are looking for inexpensive and suitable technologies. Water treatment technologies are used for three purposes i.e. water source reduction, wastewater treatment and recycling. At present, unit operations and processes are combined together to provide what is called primary, secondary and tertiary treatment. Primary treatment includes preliminary purification processes of a physical and chemical nature while secondary treatment deals with the biological treatment of wastewater. In tertiary treatment processes, wastewater (treated by primary and secondary processes) is converted into good quality water that can be used for different types of purpose, i.e. drinking, industrial, medicinal etc. supplies. In the tertiary process, up to 99% of the pollutants are removed and the water is converted into the safe quality for a specific use. In a complete water treatment plant, all these three processes are combined together for producing good quality and safe water. The complete scheme of wastewater treatment is shown in Fig. 1.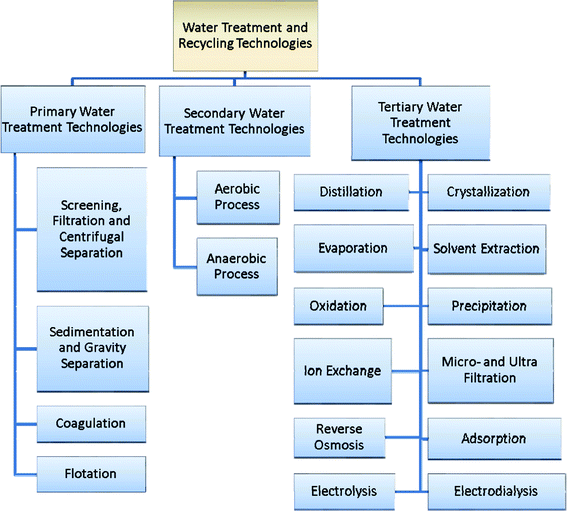 | ||
| Fig. 1 Illustration of the classification of chemical treatment and water recycling technologies. | ||
Despite the development of various technologies for water treatment and reclamation, economic, effective and rapid water treatment and reclamation at a commercial level is still a challenging problem. The management of the removed pollutants (sludge) should be kept in mind. The systematic approach of water treatment and recycling technologies involves the understanding of the technology that includes construction and operational cost, along with the maintenance and management of removed pollutants. A comparison of these wastewater treatment and reclamation technologies in terms of their applicability, suitability and costs is presented in Table 1. Water treatment and recycling technologies have been classified (Fig. 1) under the following headings.
| Wastewater technologies | Applicability | Suitability | Costs (US$ per one million liters of treated water) |
|---|---|---|---|
| a Rarely used. b Abbreviations: Sl: soluble, Ss: suspended, I: inorganics, O: organics, V: volatiles, B: biologicals, R: reclamation, T: treatment and Sr: source reduction. | |||
| Primary technologies | |||
| Screening, filtration and centrifugal | Ss & Sl IOB | RSrT | 25–450 |
| Separation | |||
| Sedimentation and gravity separation | Ss IOB | RSrT | 5–10 |
| Coagulation | Ss & Sl I | RT | 25–500 |
| Flotation | Ss IOB | RT | 5–25 |
| Secondary technologies | |||
| Aerobic | Sl & Ss O | RT | 20–200 |
| Anaerobic | Sl & Ss O | RT | 20–200 |
| Tertiary technologies | |||
| Distillation | Sl IOB | RT | 15–2000 |
| Crystallizationa | Sl IO | RSrT | 50–150 |
| Evaporationa | Sl & Ss IOB | RSrT | 15–200 |
| Solvent extraction | Sl OV | RSrT | 250–2500 |
| Oxidationa | Sl IO | RSrT | 100–2000 |
| Precipitationa | Sl IO | RT | 20–500 |
| Ion Exchange | Sl IO | RSrT | 50–200 |
| Micro- and ultra-filtration | Sl IOB | RSrT | 15–400 |
| Reverse osmosis | Sl IOB | RSrT | 20–450 |
| Adsorption | Ss & Sl IOB | RSrT | 50–150 |
| Electrolysis | Sl IO | RSrT | — |
| Electrodialysis | Sl IO | RSrT | 15–400 |
3.1 Primary water treatment technologies
3.2 Secondary water treatment technologies
3.3 Tertiary water treatment technologies
3.1 Primary water treatment technologies
In this category, water is treated at the primary level using screening, filtration, centrifugation, sedimentation, coagulation, gravity and flotation methods. Normally, these methods are used when water is highly polluted. Brief descriptions of these methods are given below.In filtration process, water is passed through a medium having fine pores. Normally, a set-up with a pore size of about 0.1 to 0.5 μm is used for this purpose. It is used for the removal of suspended solids, greases, oils, bacteria etc. Different filters, such as membranes and cartridges can be used. The filtration process can be used to remove solids of size below 100 mg l−1 and to remove oil of 25 mg l−1 which can be reduced by up to 99%. The filtration process is utilized for water treatment. Water produced by filtration is used for adsorption, ion exchange or membrane separation processes. Besides, potable water is produced by filtration systems. The cost of filtration varies from 25 to 450 US$ per million liters of treated water.3,7
Centrifugal separation is used to remove suspended non-colloidal solids (size up to 1 μm). The wastewater is applied to centrifugal devices and rotated at different speeds and the solids (sludges) are separated and discharged. The extent of separation of suspended solids is directly proportional to their densities. In addition to this, the speed of the centrifugal machine is also responsible for the removal of suspended solids. Applications include the source reduction and separation of oils and greases. The different types of centrifugal machines available and in use are solid-bowl, basket type, counter flow and counter current flow. The cost of the wastewater treatment ranges 25 to 450 US$ per million liters of treated water.3,7
3.2. Secondary water treatment technologies
Secondary water treatment includes biological routes for the removal of soluble and insoluble pollutants by microbes.3,13,14 Water is circulated in a reactor that maintains a high concentration of microbes. The microbes, usually bacterial and fungal strains, convert the organic matter into water, carbon dioxide and ammonia gas.15–19 Sometimes, the organic matter is converted into other products such as alcohol, glucose, nitrate etc. Additionally, the microbes detoxify toxic inorganic matter. The wastewater should be then free from toxic organics and inorganics. The maximum concentrations of total dissolved solids (TDS), heavy metals, cyanides, phenols and oil should not exceed by 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 2.0, 60.0, 140, and 50 mg l−1 respectively. The biological treatment includes the aerobic and anaerobic digestion of wastewater. Depending on the materials used, the cost of biological treatment varies between 20 and 200 US$ per million liters.
000, 2.0, 60.0, 140, and 50 mg l−1 respectively. The biological treatment includes the aerobic and anaerobic digestion of wastewater. Depending on the materials used, the cost of biological treatment varies between 20 and 200 US$ per million liters.
A simplified representation of aerobic decomposition is given by the following equation.
| Organic matter + O2 + Bacteria → CO2 + H2O + Bacteria + Byproducts | (1) |
The anaerobic process is represented by the following equation.
| Organic matter + Bacteria → CO2 + CH4 + Bacteria + Byproducts | (2) |
3.3. Tertiary water treatment technologies
Tertiary water treatment technologies are very important in wastewater treatment strategy as these are used to obtain safe water for human consumption. The techniques used for this purpose are distillation, crystallization, evaporation, solvent extraction, oxidation, coagulation, precipitation, electrolysis, electrodialysis, ion exchange, reverse osmosis and adsorption. These methods are described below.Photocatalysis is also one of a series of advanced oxidation processes for organic pollutant degradation.50–54 In photocatalysis, light energy from a light source (UV or solar) excites an electron from the valence band of the photocatalyst to the conduction band with a series of reactions which results in the formation of hydroxyl radicals. The hydroxyl radicals have high oxidizing potential and therefore can attack most organic pollutants causing oxidation. Various chalcogenides (oxides such as TiO2, ZnO, ZrO2, CeO2, etc. or sulfides such as CdS, ZnS, etc.) have been used as photocatalysts in the photocatalytic process and the process is found suitable for a wide range of organic pollutants.55–58 Sonolysis, i.e., use of ultrasonic waves has been used for the decolorization and degradation of organic pollutants. The mechanism proposed for the sonochemical process is usually based on the formation of short-lived radical species generated in violent cavitation events.59
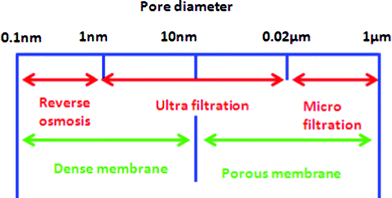 | ||
| Fig. 2 Filtration spectrum; micro-, ultra-filtration and reverse osmosis. | ||
Reverse osmosis has been used as a separation and concentration technique at macro and micro levels for the removal of large, non-polar, ionic and toxic soluble pollutants. The effectiveness and the application of RO on a large scale is based on the fact that the total dissolved solids, organic dissolved matter and bacteria can be removed by up to 99%. Reverse osmosis is used for the treatment of wastewater from sanitary waste, municipal leachates, and different industries.76,77 Besides, the rejection of bacteria, virus and other microbes is one hundred percent and, therefore, it is used in the preparation of ultra pure water for use in pharmacy, medicines and electronics. In addition, it has been used for the purpose of source reduction. RO is today's most economic process for potable water production from saline water.
The working efficiency of a RO membrane is expressed in terms of percentage rejection (PR%) or the removal ratio of solutes and expressed as:
where, CF is the molar concentration of solute in the feed water, CP is the molar concentration of solute in the permeate (filtrate) and E is the membrane working efficiency.
The performance of the membrane process is also measured by permeate flux. In desalination and wastewater treatment processes, the solvent or permeate flux is referred to the water and defined as the volume of permeate flux produced per unit membrane area for a time interval and is given by the following equation.
Separation of pollutants in RO depends on the basis of partition coefficients of solutes between water and membrane involving the free energy of interaction between water and membrane sites. The percentage of rejection is directly proportional to the number of carbon atoms or the molecular weights of organic/inorganic species but this was not found to be a complete description because of the geometry of the molecule, i.e., steric configuration and branching overshadow the former properties.
The design of reverse osmosis units plays a role to achieve efficient seawater desalination. Fig. 3 demonstrates the basic scheme of desalination by reverse osmosis in a system that does not apply energy recovery or pressure conversion devices. Membranes can be connected in series as shown in Fig. 4. The flow rate of water through the membrane is given as:
| Frate = Kf(Ppump − Ps) |
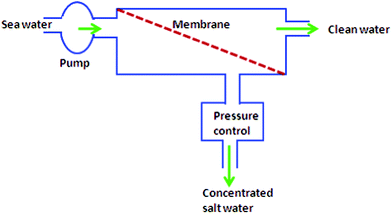 | ||
| Fig. 3 Basic design of reverse osmosis. | ||
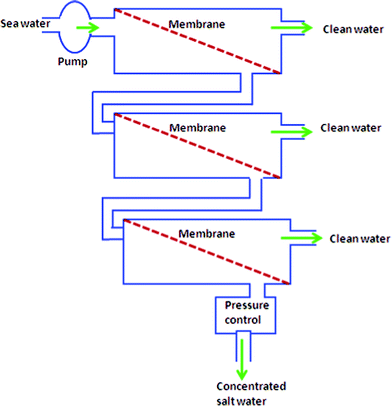 | ||
| Fig. 4 Modules of connecting membranes in series. | ||
The life of RO membranes has been considered to be two to five years depending upon the nature of the wastewater being treated. The flux and the quality of the permeate may decrease over a long period of time due to membrane fouling. After a long period of time, humic acids, bacterial slimes or hardness scales may accumulate on the RO membranes. Phenols have also been found to clog the membranes. To increase the efficiency and life of the membrane, pre-treatment is necessary to minimize the concentration of colloidal and dispersed solids. Physico-chemical coagulation with lime has been used to minimize colloids, turbidity, dispersed oil phases, metal ions and suspended matters. Sodium hydroxide solution (pH 9–11) has been used to clean RO membranes in case of silica and sulphate fouling.78,79 Besides, silica can also be removed from membranes by ion retarding resins that has a high affinity for strong acids, together with conversion of the weak acid [Si(OH)4] into much stronger acid (H2SiF6). The bacterial inhibitor solutions are circulated (to check the bacterial growth) in to RO tubules or discs before stopping the process for a long period.79
4. Selection of wastewater technologies
The selection of the treatment technologies depends on the type of the wastewater and the requirements (economy aspect). Highly polluted water having color and containing solid waste is first treated with primary and secondary processes followed by the tertiary water treatment technologies. If the BOD is negligible then a secondary process is not required. If the water is colorless without any solids and is polluted due to inorganic, organic and biological pollutants, then only tertiary water treatment is required. Generally, groundwater is polluted by toxic metal ions and anions and only tertiary water technologies are required for its treatment. Contrarily, surface water contaminated by inorganic, organic and biological pollutants requires secondary and tertiary treatment methods. Generally, wastewater is highly polluted and it may be colored with solid waste containing inorganic, organic, biological pollutants, which requires a good hyphenation of primary, secondary and tertiary treatment technologies. The choice of the tertiary water treatment technologies depends on the types of the pollutants present in the water and the optimum selection can be done by considering the above cited discussions. A brief presentation of the selection of water treatment technologies is given in Fig. 1.5. Waste management
It is clear from the above discussion that waste is generated in every water purification and recycling technology. Therefore, waste management is very important from the point of view of the environment and health. This is because, generally, waste products are toxic. The waste generated may enter water resources and contaminant it again. A large amount of solid waste is generated in screening, filtration, centrifugal separation, sedimentation and gravity separation, coagulation, flotation, aerobic process, anaerobic process, evaporation and precipitation processes. Micro- and ultra-filtration and reverse osmosis produce water containing a high concentration of pollutants. On the other hand some gases are produced in electrolysis and the anaerobic processes which are dangerous to the environment. In adsorption, the production of exhaustive adsorbents is quite high. Many methods have been developed and used for the management of waste products generated during water treatment processes. The most important waste management method is the recycling of waste products into useful products such as fertilizers, fillers, building material etc. Some toxic waste products have been burnt and their ash used as fertilizers. Suggestions have also been made to bury the waste products under ground in airtight iron or plastic containers. Nowadays, fly ash, red mud, sand etc. are being used as the useful and economic adsorbents for the removal of pollutants from water. The large quantities of these exhausted adsorbents produced have been successfully used as fillers and building materials.6. Conclusions
The water treatment technologies discussed in this article differ from each other in terms of their principles, scope of application, speed and economy. The feasibility of any water recycling technique at a commercial level depends on the cost of construction, maintenance and operation. Sludge management is also an important factor for the selection of a technology. Taking all these points into consideration, adsorption is considered the best and most universal technique as it is used for the removal of a wide variety of organic and inorganic pollutants. It is also a rapid process with a low cost of construction, maintenance and operation. Reverse osmosis is widely used and is a popular technique as the quality of water is good but the cost of construction and maintenance is comparatively high. Other techniques such as micro- and ultra-filtration, ion exchange, electrodialysis, solvent extraction, distillation etc. are used for specific purposes but their use is restricted at potable and industrial levels. The total cost of the treated water comprises the expenditure involved in the primary, secondary and tertiary process and, therefore, there is a great need to hyphenate all these process in an economic way.References
- T. Dyson, Population and food: global trends and future prospects, Routledge. London, 1996 Search PubMed.
- R. L. Droste, Theory and Practice of Water and Wastewater Treatment,John Wiley & Sons, Inc., New York, 1997 Search PubMed.
- L. B. Franklin, Wastewater Engineering: Treatment. Disposal and Reuse,McGraw Hill, Inc., New York, 1991 Search PubMed.
- V. Gaston, International Regulatory Aspects for Chemicals,Vol. I, CRC Press, Inc., New York, 1979 Search PubMed.
- D. H. Hutson and T. R. Roberts, Environmental Fate of Pesticides,Vol. 7, John Wiley & Sons, New York, 1990 Search PubMed.
- D. Z. John, Hand Book of Drinking Water Quality: Standards and Controls,Van Nostrand Reinhold, New York, 1990 Search PubMed.
- N. Nemerow and A. Dasgupta, Industrial and Hazardous Waste Treatment,Van Nostrand Reinhold, New York, 1991 Search PubMed.
- N. P. Cheremisinoff, Handbook of Water and Wastewater Treatment Technologies,Butterworth-Heinemann, Boston, 2002 Search PubMed.
- O. Marmagne and C. Coste, Am. Dyest. Rep., 1996, 85, 15–20 CAS.
- A. A. Latifossglu, G. Surucu and M. Evirgen, Water Pollut. IV: Model., Meas., Predict., 4th Int. Conf., 1997, 733–742 CAS.
- I. O. Sinev, O. P. Sinev and S. N. Linevich, Izobreteniya, 1997, 26, 369–370 Search PubMed.
- T. Clark and T. Stephenson, Environ. Technol., 1998, 19, 579–590 CrossRef CAS.
- M. T. Kato, J. A. Field and G. Lettinga, Water Sci. Technol., 1997, 36, 375–382 CrossRef CAS.
- G. A. Zinkus, W. D. Byers and W. W. Doerr, Chem. Eng. Prog., 1998, 94, 19–31 CAS.
- C. I. Pearce, J. R. Lloyd and J. T. Guthrie, Dyes Pigm., 2003, 58, 179–196 CrossRef CAS.
- Y. Fu and T. Viraraghavan, Bioresour. Technol., 2001, 79, 251–262 CrossRef CAS.
- A. R. Pendashteh, A. Fakhru'L-Razi, T. G. Chuah, A. B. D. Radiah, S. S. Madaeni and Z. A. Zurina, Environ. Toxicol., 2010, 31, 1229–1239 CAS.
- A. Joss, E. Keller, A. C. Alder, A. Göbel, C. S. McArdell, T. Ternes and H. Siegrist, Water Res., 2005, 39, 3139–3152 CrossRef CAS.
- A. Joss, S. Zabczynski, A. Göbel, B. Hoffmann, D. Löffler, C. S. McArdell, T. A. Ternes and H. Siegrist, Water Res., 2006, 40, 1686–1696 CrossRef CAS.
- B. E. Barragan, C. Costa and M. Carmen Marquez, Dyes Pigm., 2007, 75, 73–81 CrossRef CAS.
- Q. Tian and J. Chen, Journal of Dong Hua University, 2000, 17, 61–63 CAS.
- C. Fux, M. Boehler, P. Huber, I. Brunner and H. Siegrist, J. Biotechnol., 2002, 99, 295–306 CrossRef CAS.
- F. P. Van Der Zee and S. Villaverde, Water Res., 2005, 39, 1425–1440 CrossRef CAS.
- O. Bernard, Z. Hadj-Sadok, D. Dochain, A. Genovesi and J. P. Steyer, Biotechnol. Bioeng., 2001, 75, 424–438 CrossRef CAS.
- N. Bernet, N. Delgenes, J. C. Akunna, J. P. Delgenes and R. Moletta, Water Res., 2000, 34, 611–619 CrossRef CAS.
- A. M. Talarposhti, T. Donnelly and G. K. Anderson, Water Res., 2001, 35, 425–432 CrossRef CAS.
- S. Venkata Mohan, V. Lalit Babu and P. N. Sarma, Enzyme Microb. Technol., 2007, 41, 506–515 CrossRef.
- P. R. Bom, PCT Int. Appl., 1998 Search PubMed, WO 9825679 (Cl. B01D1).
- F. Vander Ham, G. J. Witkamp, J. deGrauw and G. M. van Rosmalen, Chem. Eng. Process., 1998, 37, 207–213 CrossRef CAS.
- G. A. Zinkus, W. D. Byers and W. W. Doerr, Chem. Eng. Prog., 1998, 94, 19–31 CAS.
- J. W. Ahn and J. G. Ahn, Chawn Risaikring, 1997, 6, 48–54 CAS.
- D. W. Hall and A. S. Joseph, Environ. Prog., 1990, 9, 98–105 CrossRef CAS.
- R. J. Bigda, Chem. Eng. Prog., 1995, 89, 62–66 Search PubMed.
- P. R. Gogate and A. B. Pandit, Adv. Environ. Res., 2004, 8, 501–551 CrossRef CAS.
- T. A. Ternes, J. Stüber, N. Herrmann, D. McDowell, A. Ried, M. Kampmann and B. Teiser, Water Res., 2003, 37, 1976–1982 CrossRef CAS.
- M. M. Huber, A. Göbel, A. Joss, N. Hermann, D. Löffler, C. S. McArdell, A. Ried and U. Von Gunten, Environ. Sci. Technol., 2005, 39, 4290–4299 CrossRef CAS.
- M. Pérez, F. Torrades, X. Domènech and J. Peral, Water Res., 2002, 36, 2703–2710 CrossRef.
- L. Szpyrkowicz, C. Juzzolino and S. N. Kaul, Water Res., 2001, 35, 2129–2136 CrossRef CAS.
- V. Kavitha and K. Palanivelu, Chemosphere, 2004, 55, 1235–1243 CrossRef CAS.
- J. A. Zazo, J. A. Casas, A. F. Mohedano, M. A. Gilarranz and J. J. Rodríguez, Environ. Sci. Technol., 2005, 39, 9295–9302 CrossRef CAS.
- P. Bautista, A. F. Mohedano, J. A. Casas, J. A. Zazo and J. J. Rodriguez, J. Chem. Technol. Biotechnol., 2008, 83, 1323–1338 CrossRef CAS.
- J. Yoon, Y. Lee and S. Kim, Water Sci. Technol., 2001, 44, 15–21 CAS.
- C. Comninellis, A. Kapalka, S. Malato, S. A. Parsons, I. Poulios and D. Mantzavinos, J. Chem. Technol. Biotechnol., 2008, 83, 769–776 CrossRef CAS.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS.
- J. J. Wu, C.-C. Wu, H.-W. Ma and C.-C. Chang, Chemosphere, 2004, 54, 997–1003 CrossRef CAS.
- I. A. Balciolu and M. Ötker, Chemosphere, 2003, 50, 85–95 CrossRef.
- M. Y. Ghaly, G. Härtel, R. Mayer and R. Haseneder, Waste Manage., 2001, 21, 41–47 CrossRef CAS.
- W. Gernjak, T. Krutzler, A. Glaser, S. Malato, J. Caceres, R. Bauer and A. R. Fernández-Alba, Chemosphere, 2003, 50, 71–78 CrossRef CAS.
- S. Esplugas, D. M. Bila, L. G. T. Krause and M. Dezotti, J. Hazard. Mater., 2007, 149, 631–642 CrossRef CAS.
- J. A. Herrera Melián, J. M. Doña Rodríguez, A. Viera Suárez, E. Tello Rendón, C. Valdés Do Campo, J. Arana and J. Pérez Peña, Chemosphere, 2000, 41, 323–327 CrossRef.
- K. Kabra, R. Chaudhary and R. L. Sawhney, Ind. Eng. Chem. Res., 2004, 43, 7683–7696 CrossRef CAS.
- D. Robert and S. Malato, Sci. Total Environ., 2002, 291, 85–97 CrossRef CAS.
- L. Prieto-Rodriguez, S. Miralles-Cuevas, I. Oller, A. Agüera, G. L. Puma and S. Malato, J. Hazard. Mater., 2012, 211–212, 131–137 CrossRef CAS.
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- X. Z. Li and F. B. Li, Environ. Sci. Technol., 2001, 35, 2381–2387 CrossRef CAS.
- B. Neppolian, H. C. Choi, S. Sakthivel, B. Arabindoo and V. Murugesan, J. Hazard. Mater., 2002, 89, 303–317 CrossRef CAS.
- M. Cristina Yeber, J. Rodríguez, J. Freer, N. Durán and H. D. Mansilla, Chemosphere, 2000, 41, 1193–1197 CrossRef.
- X. Z. Li, H. Liu, L. F. Cheng and H. J. Tong, Environ. Sci. Technol., 2003, 37, 3989–3994 CrossRef CAS.
- E. Naffrechoux, S. Chanoux, C. Petrier and J. Suptil, Ultrason. Sonochem., 2000, 7, 255–259 CrossRef CAS.
- Y. Nagasaki, Jpn.Kokai Tokyo Jp., 1998 Search PubMed, 1057967 [9857967] (Cl. C02F1).
- J. T. McNulty, in Ion Exchange Technology, ed. D. Naden, M. Streat, Ellis Norwood Ltd., London, 1984 Search PubMed.
- A. Dabrowski, Z. Hubicki, P. Podkościelny and E. Robens, Chemosphere, 2004, 56, 91–106 CrossRef CAS.
- T. Xu, J. Membr. Sci., 2005, 263, 1–29 CrossRef CAS.
- M. Vaca Mier, R. Lopez Callejas, R. Gehr, B. E. Jimenez Cisneros and P. J. J. Alvarez, Water Res., 2001, 35, 373–378 CrossRef CAS.
- S. Rengaraj and S.-H. Moon, Water Res., 2002, 36, 1783–1793 CrossRef CAS.
- W. Lorch, Hand Book of Water purification, 2nd ed., Ellis Horwood Ltd., London, 1981 Search PubMed.
- W. J. Koros, Chem. Eng. Prog., 1995, 91, 68–81 CAS.
- A. Bick, G. Oron, L. Gilierman and Y. Manor, Water Sci. Technol.: Water Supply, 2003, 3, 379–384 CAS.
- Z. Wu, S. Chen and Y. Zhao, Shanghai Huanjing Kexue, 1997, 16, 29–31 CAS.
- R. A. Mamigonyan and Yu. V. Gutin, Khim. Neftegazov. Mashinostr., 2003, 8, 5–7 Search PubMed.
- C. Bellona, J. E. Drewes, P. Xu and G. Amy, Water Res., 2004, 38, 2795–2809 CrossRef CAS.
- C. Fritzmann, J. Löwenberg, T. Wintgens and T. Melin, Desalination, 2007, 216, 1–76 CrossRef CAS.
- L. F. Greenlee, D. F. Lawler, B. D. Freeman, B. Marrot and P. Moulin, Water Res., 2009, 43, 2317–2348 CrossRef CAS.
- H. Ozaki and H. Li, Water Res., 2002, 36, 123–130 CrossRef CAS.
- C. Y. Tang, Q. S. Fu, A. P. Robertson, C. S. Criddle and J. O. Leckie, Environ. Sci. Technol., 2006, 40, 7343–7349 CrossRef CAS.
- B. Weber, F. Holz, in Effective Industrial Membrane Processes: Benefits and Opportunities, ed. M. K. Turner, Elsevier Publ. Ltd., Essex, 1991 Search PubMed.
- A. R. Williams, in Effective Industrial Membrane Processes: Benefits and Opportunities, ed. M. K. Turner, Elsevier Publ. Ltd., Essex, 1991 Search PubMed.
- B. Culkin and A. Plotkin, Chem. Eng. Prog., 1998, 94, 29–33 CAS.
- L. Sun, E. M. Perdue and J. F. McCarthy, Water Res., 1995, 29, 1471–1477 CrossRef CAS.
- V. K. Gupta, R. Jain and S. Varshney, J. Hazard. Mater., 2007, 142, 443–448 CrossRef CAS.
- I. Ali and V. K. Gupta, Nat. Protoc., 2007, 1, 2661–2667 CrossRef.
- D. F. Samuel M. A. Osman, Adsorption Processes for Water Treatment. Butterworths, Boston, 1987 Search PubMed.
- V. K. Gupta, A. Mittal, R. Jain, M. Mathur and S. Sikarwar, J. Colloid Interface Sci., 2006, 303, 80–86 CrossRef CAS.
- V. K. Gupta, I. Ali and V. K. Saini, J. Colloid Interface Sci., 2007, 315, 87–93 CrossRef CAS.
- V. K. Gupta, A. Mittal, L. Kurup and J. Mittal, J. Colloid Interface Sci., 2008, 319, 30–39 CrossRef CAS.
- V. K. Gupta and I. Ali, Environ. Sci. Technol., 2008, 42, 766–770 CrossRef CAS.
- V. K. Gupta, S. Agarwal and T. A. Saleh, J. Hazard. Mater., 2011, 185, 17–23 CrossRef CAS.
- V. K. Gupta, R. Jain, S. Malathi and A. Nayak, J. Colloid Interface Sci., 2010, 348, 628–633 CrossRef CAS.
- V. K. Gupta, R. Jain, M. N. Siddiqui, T. A. Saleh, S. Agarwal, S. Malati and D. Pathak, J. Chem. Eng. Data, 2010, 55, 5225–5229 CrossRef CAS.
- V. K. Gupta, A. Mittal, A. Malviya and J. Mittal, J. Colloid Interface Sci., 2009, 335(1), 24–33 CrossRef CAS.
- K. Jüttner, U. Galla and H. Schmieder, Electrochim. Acta, 2000, 45, 2575–2594 CrossRef.
- R. J. Coin and M. J. Niksa, Environ. Prog., 1996, 15, 122–127 CrossRef CAS.
- S. H. Lin and M. L. Chen, Water Res., 1997, 31, 868–876 CrossRef CAS.
- S. Zor, B. Yazici, M. Erbil and H. Galip, Water Res., 1998, 32, 579–586 CrossRef CAS.
- M. Y. A. Mollah, P. Morkovsky, J. A. G. Gomes, M. Kesmez, J. Parga and D. L. Cocke, J. Hazard. Mater., 2004, 114, 199–210 CrossRef CAS.
- M. Kobya, O. T. Can and M. Bayramoglu, J. Hazard. Mater., 2003, 100, 163–178 CrossRef CAS.
- V. Gottberg, J. M. Antonia and L. R. Siwak, Int. Desalin. Water Reuse Q., 1998, 7, 33–37 Search PubMed.
- X. Tongwen, Resour., Conserv. Recycl., 2002, 37, 1–22 CrossRef.
- A. B. Ribeiro, E. P. Mateus, L. M. Ottosen and G. Bech-Nielsen, Environ. Sci. Technol., 2000, 34, 784–788 CrossRef CAS.
- M. R. Jakobsen, J. Fritt-Rasmussen, S. Nielsen and L. M. Ottosen, J. Hazard. Mater., 2004, 106, 127–132 CrossRef CAS.
- V. K. Gupta, S. Agarwal and T. A. Saleh, Water Res., 2011, 45(6), 2207–2212 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |