Open Access Article
Open Access ArticleSynthesis of indole-linked β-cyano-enones: a pathway to indolyl-2-pyrrolones†
Nurabul
Mondal
,
Vidya
Kumari
,
Danish
Ali
and
Lokman H.
Choudhury
 *
*
Department of Chemistry, Indian Institute of Technology Patna, Bihta, Patna-801103, India. E-mail: lokman@iitp.ac.in
First published on 27th March 2025
Abstract
Herein, we report for the first time an additive- and catalyst-free dehydrogenative multicomponent reaction of arylglyoxal, malononitrile, and indoles for the one-pot synthesis of indole-linked β-cyano-enones in DMF medium. The reaction was performed at 100 °C in DMF, forming one C–C single bond and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in a single-flask. Furthermore, we developed an efficient method for the synthesis of indolyl-2-pyrrolones having a hydroxyl group-containing chiral carbon center from the β-cyano-enones using trifluoroacetic acid and water as reaction medium. The β-cyano-enones were also further transformed into indolyl-1,2-diketones via a base-mediated reaction, which yielded indolyl quinoxalines upon reaction with o-phenylenediamine (OPD).
C double bond in a single-flask. Furthermore, we developed an efficient method for the synthesis of indolyl-2-pyrrolones having a hydroxyl group-containing chiral carbon center from the β-cyano-enones using trifluoroacetic acid and water as reaction medium. The β-cyano-enones were also further transformed into indolyl-1,2-diketones via a base-mediated reaction, which yielded indolyl quinoxalines upon reaction with o-phenylenediamine (OPD).
Introduction
β-Cyano-enones are considered as important intermediates due to the presence of a nitrile group, an electron-deficient alkene, and a ketone functionality, which can act as 1,4-di electrophilic carbon sites. These 1,4-dielectrophilic compounds have great potential like common dielectrophilic synthons, such as 1,3- or 1,5-dicarbonyl compounds for the synthesis of novel organic compounds.1 β-Cyano-enones are also found in many dyes and pharmaceuticals.2 For example, compound I is used as a near-infrared dye,2a and compound II is a calcium channel blocker3 as shown in Fig. 1a. | ||
| Fig. 1 (a) Examples of β-cyano-α,β-unsaturated carbonyls in dye and pharmaceutics. (b) Examples of 2-pyrolones containing natural products. (c) Examples of bioactive indole-linked 2-pyrrolones. | ||
Nitrogen-containing heterocycles such as indole and pyrrolones are the most common scaffolds in natural products and synthetic bioactive molecules. Considering their importance, the design and development of new methods for synthesizing indole-containing and pyrrolone-containing molecules has remained an important area of research in organic synthesis. Pyrrolones are an important pharmacophore in drug design and discovery and are the building blocks of numerous bioactive molecules and natural products (Fig. 1b).4
Pyrrolone derivatives exhibit diverse medicinal properties such as antibacterial, anticancer, anti-inflammatory, and antiviral.5 Similarly, indole derivatives show antibacterial, α-amylase and monooxime inhibition, antimalarial and anti-tumor activities.6
The linking of two or more distinct heterocyclic moieties to provide a hybrid molecule with more than one pharmacophore has recently gained considerable interest. Often these hybrid molecules exhibit promising biological activities.7 Many indole-linked pyrrolone hybrids are known with notable pharmacological activities such as PIM-1 kinase inhibitor,8P. aeruginosa PBP3 inhibitor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9etc. (Fig. 1c). The literature shows that the syntheses of indole-linked-2-pyrrolones are relatively unexplored.
9etc. (Fig. 1c). The literature shows that the syntheses of indole-linked-2-pyrrolones are relatively unexplored.
Multicomponent reactions are the preferred strategy for synthesizing complex organic molecules for their virtues such as high efficiency, better atom economy, lesser waste production, and cost-effective use of time and energy.10 As part of our ongoing research11 for the synthesis of fused and functionalized heterocycles, we aimed to develop a method for the synthesis of indole linked β-cyano-enones and molecular hybrids of indole–pyrrolone from commercially available starting materials involving indole, aryl glyoxal, and malononitrile.
A comparison between recently reported works with our present methodology is shown in Scheme 1. Arai et al. reported the synthesis of β-cyano-enones from the reaction of ynones with Me3SiCN in the presence of Ni(cod)2 as a catalyst as shown in (Scheme 1A, eqn (a)).12 Lu et al. developed a palladium-catalyzed four-component reaction for the synthesis of β-cyano enones using aryl halides, calcium carbide, potassium hexacyanoferrate(II) and aroyl chlorides as shown in Scheme 1A, eqn (b).13 Likewise, Liu et al. reported the synthesis of β-cyano enones from the enaminone and TMSCN using molecular iodine as a catalyst (Scheme 1A, eqn (c)).14
 | ||
| Scheme 1 Comparison of our methodology with some reported methods for the synthesis of (A) β-cyano-enones. (B) Substituted 2-pyrrolones. | ||
Similar to β-cyano-enones, a few recent methods for the preparation of pyrrolones are shown in Scheme 1B. Baidya and co-workers reported the synthesis of densely functionalized pyrrolones by reacting α-amino ketones with α-keto esters at room temperature in the presence of copper/organo cooperative catalysts (Scheme 1B, eqn (a)).15 Huang et al. reported a method for the synthesis of nitro-substituted pyrrolones using a chiral copper catalyst involving asymmetric 1,4-Michael addition reactions (Scheme 1B, eqn (b)).16 Sujatha et al. synthesized highly functionalized pyrrolones by Cu-catalyzed azirine–alkyne ring-expansion reaction in the presence of molecular oxygen (Scheme 1B, eqn (c)).17 Likewise, Mansaray et al. reported indole-linked pyrrolidone synthesis by Michael's addition reaction of 2,3-dioxopyrrolidine with indole in aqueous media (Scheme 1B, eqn (d)).18
To the best of our knowledge, to date, there is no report for the efficient synthesis of indole-linked β-cyano-enones and a straightforward approach for accessing indole-linked densely substituted pyrrolones without involving any metal or hazardous reagents. Herein, we report for the first time indole-linked highly substituted pyrrolone synthesis proceeding via an acid-catalyzed cyclization of our synthesized multicomponent β-cyano-enones.
Results and discussion
We began our investigation using the model substrates phenylglyoxal monohydrate (1a), indole (2a), and malononitrile (3a) (Table 1). In a DMF medium keeping the reaction temperature at 100 °C, an indole-substituted β,β-dicyano-enone (5a) was formed with an 83% yield within 8 hours (Table 1, entry 1). Interestingly, when the reaction was performed for 4 h, we observed the desired product 5a with only a 29% yield, along with the corresponding three-component indole-substituted β,β-dicyanoketone (4a) as the major product (Table 1, entry 2). An attempt to perform this reaction at room temperature failed to yield the desired product 5a. Instead, it produced 4a with an 80% yield, along with a trace amount of the pseudo-three-component side product 4a′ (Table 1, entry 3). Next, the reaction at 50 °C in DMF produced only tri-substituted methane 4a with 82% of yield (Table 1, entry 4). We also realized that the compound 4a on heating at 100 °C in DMF for 5 h converted to our desired product 5a. When the reaction was conducted at a higher temperature of 130 °C in DMF, the reaction time decreased to 5 h, though the observed yield dropped to 75% (Table 1, entry 5). After this, different organic solvents were screened by replacing DMF for this reaction. Replacing DMF by DMSO we observed the formation of a mixture of two products 4a (30%) and 5a (51%) (Table 1, entry 6). Solvents like acetonitrile, EtOH, and EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) did not provide 5a even after 8 h of heating at 80 °C. In these cases, the formation of 4a and 4a′ were observed (Table 1, entries 7–9). Using PEG 400 instead of DMF, only a trace amount of 5a was observed (Table 1, entry 10). 2-Pyrrolidone instead of DMF produced 5a with 74% of yield (entry 11). Further, the effect of acid and base catalysts was investigated by performing the reaction in the presence of 20 mol% of FeCl3, NH2SO3H, and Cs2CO3. These attempts did not provide expected yields (Table 1, entries 12–14).
1) did not provide 5a even after 8 h of heating at 80 °C. In these cases, the formation of 4a and 4a′ were observed (Table 1, entries 7–9). Using PEG 400 instead of DMF, only a trace amount of 5a was observed (Table 1, entry 10). 2-Pyrrolidone instead of DMF produced 5a with 74% of yield (entry 11). Further, the effect of acid and base catalysts was investigated by performing the reaction in the presence of 20 mol% of FeCl3, NH2SO3H, and Cs2CO3. These attempts did not provide expected yields (Table 1, entries 12–14).
| Entry | Deviation from standard conditions | Yieldb |
|---|---|---|
| 4a/5a/4a′ | ||
| a Reaction conditions: 1a (0.25 mmol), 2a (0.25 mmol), and 3a (0.25 mmol) in 2 mL solvent. b Isolated yield. c Reaction continued for 5 h. d Heated at 80 °C instead of 100 °C. | ||
| 1 | None | Nil/83/nil |
| 2 | 4 h instead of 8 h | 60/29/nil |
| 3 | Room temp. instead of 100 °C | 80/nil/trace |
| 4 | 50 °C instead of 100 °C | 82/nil/trace |
| 5c | 130 °C instead of 100 °C | Nil/75/nil |
| 6 | DMSO instead of DMF | 30/51/nil |
| 7d | ACN instead of DMF | 55/nil/20 |
| 8d | EtOH instead of DMF | 52/nil/23 |
| 9d | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of DMF 1) instead of DMF |
55/nil/21 |
| 10 | PEG 400 instead of DMF | 65/trace/nil |
| 11 | 2-Pyrrolidone instead of DMF | Nil/74/nil |
| 12 | Presence of 20 mol% FeCl3 | Nil/71/nil |
| 13 | Presence of 20 mol% NH2SO3H | 20/60/nil |
| 14 | Presence of 20 mol% Cs2CO3 | Nil/74/nil |
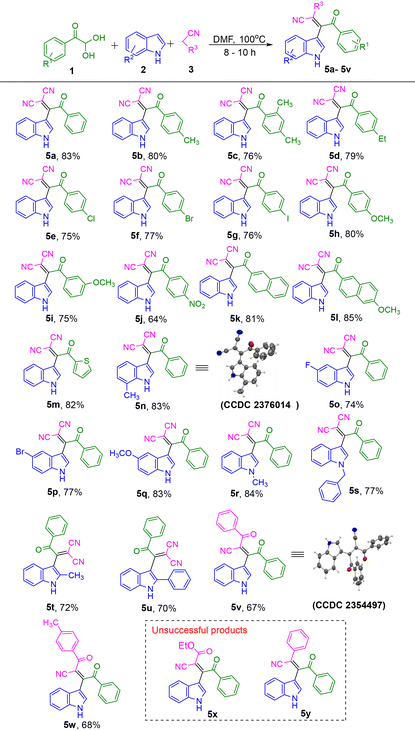
Using the optimized reaction conditions for the synthesis of β-cyano-enones (Table 1, entry 1), the scope and generality of this method was further investigated by varying the arylglyoxal, indole, and malononitrile. The results are summarized in Table 2. Arylglyoxals with para methyl, ortho, para dimethyl and para ethyl substituents produced the desired products 5b, 5c and 5d with yields of 80%, 76% and 79% respectively. Likewise, halogens (–Cl, –Br, and –I) substituted arylglyoxal reacted efficiently and produced the desired products 5e, 5f, and 5g in good yields of 75%, 77%, and 76% respectively. para-Methoxy phenylglyoxal provided the corresponding product 5h with a very good yield of 80%. When the aryl glyoxal had –OCH3 group at meta position, it produced 5i with 75% of yield. In contrast, an electron-withdrawing –NO2 group at the para position of phenylglyoxal gave a moderate yield of 64% for product 5j. Bulkier arylglyoxals, such as 2-naphthyl glyoxal and 6-methoxy-2-naphthyl glyoxal, afforded the corresponding products 5k and 5l with very good yields of 81% and 85%, respectively. Then, to check the feasibility of this reaction for heteroaryl glyoxal we performed the reaction with 2-thiophene glyoxal hydrate. It provided the product 5m with 82% yield.
Next, the effect of substituents on the indole was examined. Products 5n, 5o, 5p, and 5q having Me, –F, –Br, and –OMe substituent in the indole were prepared in good to very good yields. Likewise, indoles having, N-Me, and N-Bn substituents also provided the corresponding desired products 5r and 5s in good to very good yields. 2-Methyl indole and 2-Ph indole also produced the corresponding products 5t and 5u in good yields. Interestingly, the reaction worked even after replacing malononitrile with benzoyl acetonitrile and its derivatives. Products 5v and 5w were obtained at 67% and 68% respectively. It is noteworthy to mention that replacing malononitrile by ethyl cyanoacetate or benzyl nitrile did not provide the corresponding expected β-cyanoenones 5x and 5y. All the synthesized products were fully characterized using 1H, 13C{1H} NMR and HRMS. In addition to these, the structure of the compounds 5n and 5v were further confirmed by single crystal XRD analysis.
Next, we turned our attention to utilizing these β-cyano-enones 5 for the synthesis of indole-linked 2-pyrrolones. Initially, we optimized the reaction conditions using different reaction conditions (Table 3). The optimum condition was achieved using TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the reaction medium keeping the reaction at 80 °C for 3 h. At room temperature, instead of heating, we observed 20% yield of 6a after continuing the reaction for 24 h (Table 3, entry 2). In the TFA/H2O (1
1) as the reaction medium keeping the reaction at 80 °C for 3 h. At room temperature, instead of heating, we observed 20% yield of 6a after continuing the reaction for 24 h (Table 3, entry 2). In the TFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system, we got an 80% yield of 6a (Table 3, entry 3). When the reaction was conducted in only TFA, the observed yield dropped to 40% (Table 3 entry 4). The reaction did not work in H2O and 50% AcOH in H2O solvent (Table 3, entries 5 and 6). However, in AcOH/H2O in a 2
1) solvent system, we got an 80% yield of 6a (Table 3, entry 3). When the reaction was conducted in only TFA, the observed yield dropped to 40% (Table 3 entry 4). The reaction did not work in H2O and 50% AcOH in H2O solvent (Table 3, entries 5 and 6). However, in AcOH/H2O in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the trace amount of 6a was obtained after continuing the reaction at 120 °C for 24 h. In only AcOH, instead of TFA/H2O (2
1 ratio, the trace amount of 6a was obtained after continuing the reaction at 120 °C for 24 h. In only AcOH, instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), product 6a was observed with a 25% of yield after keeping the reaction at 120 °C for 24 h (Table 3, entry 8).
1), product 6a was observed with a 25% of yield after keeping the reaction at 120 °C for 24 h (Table 3, entry 8).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Deviation from standard condition | Yieldb |
|---|---|---|
| a Reaction conditions: 5a (0.25 mmol) in 2 mL solvent. b Isolated yield of 6a. c Reaction continued for 24 h. d Heated at 120 °C instead of 80 °C. | ||
| 1 | None | 86 |
| 2c | Room temperature instead of 80 °C | 20 |
| 3 | TFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of TFA/H2O (2 1) instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 |
| 4 | TFA instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40 |
| 5c | H2O instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Nil |
| 6c | AcOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of TFA/H2O (2 1) instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Nil |
| 7c,d | AcOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of TFA/H2O (2 1) instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Trace |
| 8c,d | AcOH instead of TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 |
Initially, 5-hydroxy-4-(1H-indol-3-yl)-2-oxo-5-phenyl-2,5-dihydro-1H-pyrrole-3-carbonitrile 6a was synthesized using optimum reaction conditions. Next, substrate scope was studied by using different substituents at phenyl and indole rings (Table 4). 4-Ethyl substituent at the para position of phenyl ring produced 6b with 87% of yield. Halogen groups such as –Cl and –I at the para position of the phenyl ring produced 6c and 6d with 80% and 82% of yield respectively. The electron-releasing and withdrawing groups such as –OCH3 and –NO2 substituted products 6e and 6f were obtained with 84% and 80% of yield respectively. N-CH3 and N-Bn substituted indolyl products 6g and 6h were observed with 88% and 81% yield. 7-Me-indolyl product 6i was found with 85% of yield. 5-F, 6-Cl and 5-Br indolyl-pyrrolones 6j, 6k and 6l were obtained with 87%, 80% and 86% of yield respectively. 5-OCH3 indolyl-pyrrolone 6m was found with 84% of yield. It is noteworthy to mention, that when β-cyano-enones 5v was tried in this process, the intended result did not occur. Since there is just one –CN group in trans to the benzoyl group (5v XRD structure), the cyclization did not proceed. All the products were fully characterized by 1H NMR, 13C{1H} NMR and HRMS. The exact structure of compound 6a was further confirmed by single-crystal XRD analysis.
After, preparing the set of indolyl-2-pyrrolone derivatives in an acidic medium we performed a reaction of β-cyano-enone 5a with an aqueous NaOH solution, and the reaction was performed under reflux conditions. Within 4 h, the 1,2-diketone product 7a was obtained with 76% of yield. Next, using this strategy, other indole-linked 1,2-diketones 7b–7d were prepared in good yields (Table 5). It is interesting to note that in this process an alkene has broken to generate the ketone functional group in the presence of a simple base.
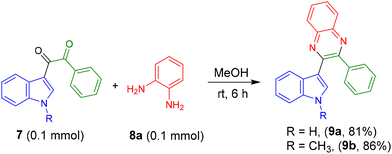
Next, product 7 was reacted with o-phenylenediamine (OPD) 8a in MeOH solvent to get the indolyl quinoxaline 9a in 81% yield (Scheme 2). Similarly, product 9b was synthesized in 86% yield.
After the synthesis of functionalized pyrrolone and quinoxaline with the synthesized multicomponent product β-cyano-enones, we aimed for the further functionalization of the indole of the synthesized β-cyano-enones. Upon reacting the β-cyano-enone 5a with benzyl bromide in DMF at room temperature. It provided N-benzylated indole linked β-cyano-enone 5s in 88% yield (Scheme 3).
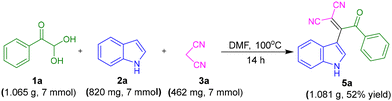
A gram-scale synthesis for 5a was performed employing 1.065 g (7 mmol) 1a, 820 mg (7 mmol) 2a with 462 mg (7 mmol) 3a. The target product 5a was obtained with a 52% yield, confirming the viability of the protocol for large-scale application (Scheme 4).
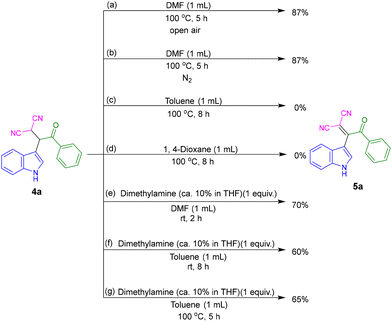
DMF is a versatile solvent that shows extraordinary properties in many reactions. It is also used as a reagent, a catalyst, and a stabilizer which can provide various units such as HCO2, O, CO, H˙, H−, NMe2, CONMe2, Me, CHO, etc.19 To understand the role of the solvent in our multicomponent reaction, a series of control experiments were conducted. For this, the conversion of β,β-dicyano-ketone (4a) to β,β-dicyano-enone (5a) was chosen. The reactions were performed using a 10 mL sealed tube. In DMF solvent, both under open air and nitrogen (N2) atmosphere at 100 °C, the desired product 5a was obtained with an 87% yield (Fig. 2a and b). These results suggest that dehydrogenative oxidation is not occurring via air oxidation. In contrast, when the reaction was performed in toluene or 1,4-dioxane, product 5a was not observed (Fig. 2c and d), indicating that thermal conditions alone are insufficient for the conversion. However, when the reaction was conducted in DMF in the presence of 1 equiv. of dimethylamine, 5a was produced even at room temperature with a 70% yield (Fig. 2e). To further investigate the role of dimethylamine, a reaction was performed in toluene in the presence of dimethylamine, resulting in the unexpected formation of 5a (Fig. 2f and g). At room temperature, after 8 h, a yield of 60% of 5a was observed (Fig. 2f). When the reaction was performed at 100 °C, the yield increased to 65% (Fig. 2g).
Based on the above observations and literature reports,20 we propose that DMF undergoes partial decomposition under heating conditions to generate dimethylamine and carbon monoxide, thereby creating weakly basic conditions. The decomposition of DMF at 100 °C is further supported by gas chromatography (GC) analysis. The detection of carbon monoxide in the GC analysis, shown in Fig. S5 of the ESI,† provides evidence for the thermal decomposition of DMF.
We have proposed a mechanism in Scheme 5 based on our observation and the literature report.21 We believe, initially, Knoevenagel condensation between phenylglyoxal 1a and malononitrile 3a takes place to provide intermediate A. Then Michael-type reaction with indole 2a provides the product 4a. Product 4a was isolated and fully characterized. The compound 4a upon heating at 100 °C in DMF solvent provides highly conjugated product 5a (Scheme 5a). The liberated H2 gas was detected by GC (Fig. S3 in ESI†). Based on the literature19,20 and preliminary experiments (Fig. 2), it is believed that the trace amount of dimethylamine is generated from the DMF under heating conditions. It makes the basic environment for abstracting the most acidic proton from the 4a followed by hydride elimination to form the desired product 5a. Based on the literature on acid-catalyzed selective conversion of nitriles to amides,22 we have proposed the mechanism for forming compound 6a (Scheme 5b). Adding trifluoroacetate (TFA) to one of the nitriles followed by hydration reaction produces intermediate D. Which upon intramolecular cyclization provides desired densely substituted pyrrolone 6a.
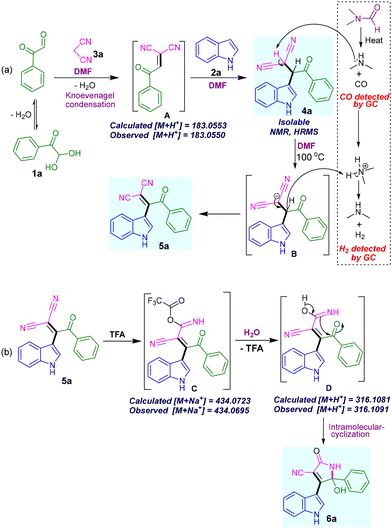 | ||
| Scheme 5 Proposed reaction mechanisms. (a) Synthesis of β,β-dicyano-enone 5a. (b) Synthesis of indolyl-2-pyrrolones 6a from β,β-dicyano-enone 5a. | ||
Conclusions
In summary, we have developed a metal-free dehydrogenative multicomponent reaction for synthesizing indole-linked β-cyano-enones using readily available starting materials: arylglyoxal, indole, and malononitrile. These β-cyano-enones can be further transformed into medicinally relevant indole-linked 2-pyrrolones featuring a hydroxyl group containing chiral carbon centre. The reaction conditions are straightforward, with a broad substrate scope. Additionally, we have established a method for converting the β-cyano-enones into indole-substituted 1,2-diketones, which can subsequently be transformed into indole-linked quinoxalines.Experimental section
General information
All the starting materials were procured from commercial suppliers such as TCI, Sigma Aldrich, Alfa Aesar, bldpharm, and Chemscene and used without further purification. Anhydrous sodium sulfate was used to dry organic extracts. Solvents were removed by using a rotary evaporator under reduced pressure. Heating reactions were performed using a silicone oil bath using a heating cum magnetic stirrer. The progress of reactions was monitored by TLC. Melting points were determined using the automated SRS EZ-Melt instrument. 1H NMR spectra were recorded using Bruker 400 MHz or JEOL 500 MHz spectrometers, while 13C{1H} NMR spectra were obtained at 100 MHz or 125 MHz in DMSO-d6 or CDCl3. Chemical shifts are referenced in δ ppm downfield. Multiplicities were designated as follows: dd (doublet of doublets), td (triplet of doublets), s (singlet), d (doublet), t (triplet), m (multiplet), bs (broad singlet), and coupling constants (J, in Hz) were indicated. HRMS data were acquired utilizing a BRUKER Impact HD mass spectrometer (ESI, positive mode) or SCIEX Model-X500R QTOF. Column chromatographic purifications were performed using SRL silica gel (100–200 mesh). Single crystal XRD was recorded using BRUKER AXS D8 quest system or BRUKER D8 venture diffractometer system. Gas Chromatography (GC) was performed using Centurion Scientific, 5800 Gas Chromatograph.Experimental procedure
General experimental procedure for the synthesis of 5a
Phenylglyoxal monohydrate 1a (38 mg, 0.25 mmol), indole 2a (29 mg, 0.25 mmol), and malononitrile 3a (17 mg, 0.25 mmol) were taken in a 10 mL round bottom flask. Then 2 mL of DMF was added and stirred at 100 °C for 8 h. The progress of the reaction was monitored using TLC. After completion of the reaction, the reaction mixture was quenched with ice-cold water and extracted with ethyl acetate (3 × 20 mL). Then combined organic layer was washed with brine solution and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using a rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as the stationary phase and different ratios of hexane/ethyl acetate mixture as eluent. The other derivatives 5b–5w were made using similar methods.General experimental procedure for the synthesis of 6a
In a 10 mL glass sealed tube, 1.0 mL TFA/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mix solvent was cooled by placing an ice bath. β,β-Dicyano-enones 5a (0.1 mmol, 30 mg) was added keeping the reaction temperature at 0 °C. Then the reaction tube was stirred for 3 h keeping the reaction temperature at 80 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into 20 mL of water and neutralized by saturated NaHCO3 solution. Then the product was extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using a rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as the stationary phase and different ratios of hexane/ethyl acetate mixture as eluent. The other derivatives 6b–6m were made using similar methods.
1) mix solvent was cooled by placing an ice bath. β,β-Dicyano-enones 5a (0.1 mmol, 30 mg) was added keeping the reaction temperature at 0 °C. Then the reaction tube was stirred for 3 h keeping the reaction temperature at 80 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into 20 mL of water and neutralized by saturated NaHCO3 solution. Then the product was extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using a rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as the stationary phase and different ratios of hexane/ethyl acetate mixture as eluent. The other derivatives 6b–6m were made using similar methods.
General experimental procedure for the synthesis of 7a
In a 10 mL of glass sealed tube 4 mg (1 equiv.) of NaOH was dissolved in 1 mL of distilled water. Then β,β-dicyano-enones 5a (0.1 mmol, 30 mg) was added. The reaction mixture was heated at 100 °C for 4 h. Progress of the reaction was monitored by TLC. After completion of the reaction, reaction mixture was poured in 20 mL of water. Then the product was extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as stationary phase and hexane/ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as eluent. The other derivatives 7b–7d were made using similar methods.
1) mixture as eluent. The other derivatives 7b–7d were made using similar methods.
General experimental procedure for the synthesis of indolyl-quinoxalines (9a)
In a 10 mL of glass sealed tube, 7a (0.1 mmol, 25 mg) and o-phenylenedimine 8a (OPD) (0.1 mmol, 11 mg) were dissolved in 1 mL of MeOH. Then the reaction mixture was stirred at room temperature for 6 h. Progress of the reaction was monitored by TLC. After completion of the reaction, reaction mixture was poured in 20 mL of water. Then the product was extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as stationary phase and hexane/ethyl acetate (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) mixture as eluent. The other derivative 9b was also made using similar methods.
0.5) mixture as eluent. The other derivative 9b was also made using similar methods.
Procedures for N-benzylation of β-cyano-enone (5a)
In a 10 mL of glass sealed tube 5 mg (1.4 equiv.) of NaH (63–67% oil dispersion) and 30 mg (0.1 mmol) of 5a were dissolved in 1 mL of DMF. Then 14 μL (0.12 mmol) of benzyl bromide was added and started stirring for 2 h. Progress of the reaction was monitored by TLC. After completion of the reaction, reaction mixture was poured in 20 mL of ice cold water. Then the product was extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as stationary phase and hexane/ethyl acetate (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) mixture as eluent.
1.5) mixture as eluent.
General procedures for the gram-scale synthesis of compound 5a
Phenylglyoxal monohydrate 1a (1.065 g, 7 mmol), indole 2a (820 mg, 7 mmol), and malononitrile 3a (462 mg, 7 mmol) were taken in a 100 mL round bottom flask. Then 30 mL of DMF was added and stirred at 100 °C for 14 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was quenched with ice-cold water and extracted with ethyl acetate (3 × 80 mL). Then combined organic layer was washed with brine solution and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure using a rotary evaporator and collected for reuse, and the solid crude product was purified by column chromatography using silica gel as stationary phase and hexane/ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture as eluent.
2) mixture as eluent.
Characterization data
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). Yield 61 mg (82%); light brown solid; mp 156–158 °C. 1H NMR (500 MHz, CDCl3) δ 8.44 (s, 1H), 7.93 (d, J = 7.2 Hz, 2H), 7.74 (d, J = 8.1 Hz, 1H), 7.50 (t, J = 7.4 Hz, 1H), 7.40 (d, J = 7.1 Hz, 1H), 7.35 (t, J = 8.5 Hz, 2H), 7.29–7.23 (m, 2H), 7.22 (d, J = 2.8 Hz, 1H), 5.48 (d, J = 7.7 Hz, 1H), 4.61 (d, J = 7.7 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 193.2, 136.5, 134.3, 134.1, 129.1, 128.9, 125.2, 124.9, 123.6, 121.4, 118.1, 112.6, 112.4, 112.1, 107.1, 47.2, 26.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H14N3O+, 300.1131; found, 300.1160.
1.5). Yield 61 mg (82%); light brown solid; mp 156–158 °C. 1H NMR (500 MHz, CDCl3) δ 8.44 (s, 1H), 7.93 (d, J = 7.2 Hz, 2H), 7.74 (d, J = 8.1 Hz, 1H), 7.50 (t, J = 7.4 Hz, 1H), 7.40 (d, J = 7.1 Hz, 1H), 7.35 (t, J = 8.5 Hz, 2H), 7.29–7.23 (m, 2H), 7.22 (d, J = 2.8 Hz, 1H), 5.48 (d, J = 7.7 Hz, 1H), 4.61 (d, J = 7.7 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 193.2, 136.5, 134.3, 134.1, 129.1, 128.9, 125.2, 124.9, 123.6, 121.4, 118.1, 112.6, 112.4, 112.1, 107.1, 47.2, 26.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H14N3O+, 300.1131; found, 300.1160.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). White solid; mp 212 °C. 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 7.2 Hz, 2H), 8.03 (s, 2H), 7.56 (d, J = 7.9 Hz, 2H), 7.52 (t, J = 7.4 Hz, 1H), 7.41 (t, J = 7.6 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 7.18 (t, J = 7.6 Hz, 2H), 7.08 (t, J = 7.1 Hz, 2H), 6.92 (s, 2H), 6.52 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 198.7, 137.1, 136.7, 133.0, 128.9, 128.8, 126.8, 124.1, 122.4, 119.8, 119.1, 114.4, 111.5, 42.2.
2). White solid; mp 212 °C. 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 7.2 Hz, 2H), 8.03 (s, 2H), 7.56 (d, J = 7.9 Hz, 2H), 7.52 (t, J = 7.4 Hz, 1H), 7.41 (t, J = 7.6 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 7.18 (t, J = 7.6 Hz, 2H), 7.08 (t, J = 7.1 Hz, 2H), 6.92 (s, 2H), 6.52 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 198.7, 137.1, 136.7, 133.0, 128.9, 128.8, 126.8, 124.1, 122.4, 119.8, 119.1, 114.4, 111.5, 42.2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 62 mg (83%); yellow solid; mp 200–202 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.99 (s, 1H), 8.73 (s, 1H), 8.08 (d, J = 5 Hz, 2H), 7.77 (t, J = 10 Hz, 1H), 7.61–7.58 (m, 3H), 7.26–7.21 (m, 2H), 7.11 (t, J = 10 Hz, 1H) ppm. 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 164.1, 137.1, 136.0, 135.3, 133.2, 129.8, 129.7, 124.1, 123.8, 123.1, 119.7, 115.1, 113.9, 113.8, 108.1, 69.9 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C19H11N3NaO+, 320.0794; found, 320.0824.
2). Yield 62 mg (83%); yellow solid; mp 200–202 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.99 (s, 1H), 8.73 (s, 1H), 8.08 (d, J = 5 Hz, 2H), 7.77 (t, J = 10 Hz, 1H), 7.61–7.58 (m, 3H), 7.26–7.21 (m, 2H), 7.11 (t, J = 10 Hz, 1H) ppm. 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 164.1, 137.1, 136.0, 135.3, 133.2, 129.8, 129.7, 124.1, 123.8, 123.1, 119.7, 115.1, 113.9, 113.8, 108.1, 69.9 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C19H11N3NaO+, 320.0794; found, 320.0824.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 62 mg (80%); yellow solid; mp 215–217 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.73 (s, 1H), 7.96 (d, J = 10 Hz, 2H), 7.58 (d, J = 10 Hz, 1H), 7.40 (d, J = 5 Hz, 2H), 7.24 (t, J = 10 Hz, 1H), 7.20 (d, J = 10 Hz, 1H), 7.10 (t, J = 10 Hz, 1H), 2.38 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.4, 164.4, 147.2, 137.1, 135.2, 130.9, 130.4, 129.9, 124.1, 123.9, 123.1, 119.7, 115.2, 114.0, 113.8, 108.2, 69.7, 21.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1154.
2). Yield 62 mg (80%); yellow solid; mp 215–217 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.73 (s, 1H), 7.96 (d, J = 10 Hz, 2H), 7.58 (d, J = 10 Hz, 1H), 7.40 (d, J = 5 Hz, 2H), 7.24 (t, J = 10 Hz, 1H), 7.20 (d, J = 10 Hz, 1H), 7.10 (t, J = 10 Hz, 1H), 2.38 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.4, 164.4, 147.2, 137.1, 135.2, 130.9, 130.4, 129.9, 124.1, 123.9, 123.1, 119.7, 115.2, 114.0, 113.8, 108.2, 69.7, 21.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1154.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). Yield 62 mg (76%); yellow solid; mp 195–197 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.72 (s, 1H), 7.71 (d, J = 8.1 Hz, 1H), 7.59 (d, J = 8.1 Hz, 1H), 7.31 (s, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.1 Hz, 2H), 2.71 (s, 3H), 2.33 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.6, 165.6, 145.9, 141.2, 136.9, 135.2, 133.6, 129.2, 127.4, 124.0, 123.9, 122.9, 119.7, 115.3, 113.9, 113.7, 108.5, 69.5, 21.7, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H16N3O+, 326.1288; found, 326.1287.
1.5). Yield 62 mg (76%); yellow solid; mp 195–197 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.72 (s, 1H), 7.71 (d, J = 8.1 Hz, 1H), 7.59 (d, J = 8.1 Hz, 1H), 7.31 (s, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.1 Hz, 2H), 2.71 (s, 3H), 2.33 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.6, 165.6, 145.9, 141.2, 136.9, 135.2, 133.6, 129.2, 127.4, 124.0, 123.9, 122.9, 119.7, 115.3, 113.9, 113.7, 108.5, 69.5, 21.7, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H16N3O+, 326.1288; found, 326.1287.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 64 mg (79%); yellow solid; mp 197–199 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.72 (s, 1H), 7.98 (d, J = 10 Hz, 2H), 7.58 (d, J = 10 Hz, 1H), 7.43 (d, J = 10 Hz, 2H), 7.26–7.21 (m, 2H), 7.11 (t, J = 10 Hz, 1H), 2.68 (q, J = 10 Hz, 2H), 1.17 (t, J = 10 Hz, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.4, 164.4, 152.9, 137.1, 135.2, 131.1, 130.0, 129.2, 124.1, 123.8, 123.1, 119.7, 115.2, 114.0, 113.8, 108.2, 69.7, 28.4, 14.8 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H16N3O+, 326.1288; found, 326.1295.
2). Yield 64 mg (79%); yellow solid; mp 197–199 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.98 (s, 1H), 8.72 (s, 1H), 7.98 (d, J = 10 Hz, 2H), 7.58 (d, J = 10 Hz, 1H), 7.43 (d, J = 10 Hz, 2H), 7.26–7.21 (m, 2H), 7.11 (t, J = 10 Hz, 1H), 2.68 (q, J = 10 Hz, 2H), 1.17 (t, J = 10 Hz, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.4, 164.4, 152.9, 137.1, 135.2, 131.1, 130.0, 129.2, 124.1, 123.8, 123.1, 119.7, 115.2, 114.0, 113.8, 108.2, 69.7, 28.4, 14.8 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H16N3O+, 326.1288; found, 326.1295.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 62 mg (75%); yellow solid; mp 213–215 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.71 (s, 1H), 8.10 (d, J = 8.7 Hz, 2H), 7.67 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 191.9, 163.3, 141.2, 137.1, 135.5, 131.9, 131.6, 130.0, 124.2, 123.8, 123.2, 119.7, 115.1, 113.9, 113.8, 107.9, 70.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11ClN3O+, 332.0585; found, 332.0582.
2). Yield 62 mg (75%); yellow solid; mp 213–215 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.71 (s, 1H), 8.10 (d, J = 8.7 Hz, 2H), 7.67 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 191.9, 163.3, 141.2, 137.1, 135.5, 131.9, 131.6, 130.0, 124.2, 123.8, 123.2, 119.7, 115.1, 113.9, 113.8, 107.9, 70.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11ClN3O+, 332.0585; found, 332.0582.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 72 mg (77%); yellow solid; mp 217–219 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.72 (s, 1H), 8.01 (d, J = 8.7 Hz, 2H), 7.82 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 7.2 Hz, 1H), 7.26 (t, J = 8.2 Hz, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.2, 163.3, 137.1, 135.5, 132.9, 132.3, 131.6, 130.7, 124.2, 123.8, 123.2, 119.7, 115.1, 113.96, 113.86, 107.9, 70.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11BrN3O+, 376.0080; found, 376.0104.
2). Yield 72 mg (77%); yellow solid; mp 217–219 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.72 (s, 1H), 8.01 (d, J = 8.7 Hz, 2H), 7.82 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 7.2 Hz, 1H), 7.26 (t, J = 8.2 Hz, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.2, 163.3, 137.1, 135.5, 132.9, 132.3, 131.6, 130.7, 124.2, 123.8, 123.2, 119.7, 115.1, 113.96, 113.86, 107.9, 70.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11BrN3O+, 376.0080; found, 376.0104.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 80 mg (76%); yellow solid; mp 248–250 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.01 (s, 1H), 8.72 (s, 1H), 8.00 (d, J = 8.6 Hz, 2H), 7.82 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.1 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.6, 163.3, 138.8, 137.1, 135.5, 132.5, 131.1, 124.1, 123.7, 123.2, 119.6, 115.1, 113.9, 113.8, 107.9, 106.2, 69.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11IN3O+, 423.9941; found, 423.9943.
2). Yield 80 mg (76%); yellow solid; mp 248–250 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.01 (s, 1H), 8.72 (s, 1H), 8.00 (d, J = 8.6 Hz, 2H), 7.82 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.1 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.6, 163.3, 138.8, 137.1, 135.5, 132.5, 131.1, 124.1, 123.7, 123.2, 119.6, 115.1, 113.9, 113.8, 107.9, 106.2, 69.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11IN3O+, 423.9941; found, 423.9943.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 65 mg (80%); yellow solid; mp 215–217 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.95 (s, 1H), 8.72 (s, 1H), 8.03 (d, J = 9.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 1H), 7.25–7.21 (m, 2H), 7.11–7.09 (m, 3H), 3.85 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 190.9, 165.3, 164.6, 137.0, 135.2, 132.5, 126.2, 124.0, 123.9, 123.0, 119.8, 115.3, 115.1, 114.1, 113.7, 108.2, 69.6, 55.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O2+, 328.1081; found, 328.1080.
2). Yield 65 mg (80%); yellow solid; mp 215–217 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.95 (s, 1H), 8.72 (s, 1H), 8.03 (d, J = 9.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 1H), 7.25–7.21 (m, 2H), 7.11–7.09 (m, 3H), 3.85 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 190.9, 165.3, 164.6, 137.0, 135.2, 132.5, 126.2, 124.0, 123.9, 123.0, 119.8, 115.3, 115.1, 114.1, 113.7, 108.2, 69.6, 55.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O2+, 328.1081; found, 328.1080.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). Yield 61 mg (75%); yellow solid; mp 219–221 °C. 1H NMR (500 MHz, DMSO-d6 + CDCl3) δ 12.98 (s, 1H), 8.72 (s, 1H), 7.62–7.57 (m, 3H), 7.49 (t, J = 8.0 Hz, 1H), 7.35 (dd, J = 8.2, 2.7 Hz, 1H), 7.27–7.22 (m, 2H), 7.12 (t, J = 7.7 Hz, 1H), 3.83 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.6, 163.9, 159.9, 137.0, 135.2, 134.5, 130.9, 124.0, 123.8, 123.0, 122.8, 121.9, 119.7, 115.1, 113.9, 113.7, 113.4, 108.1, 69.9, 55.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C20H13N3O2Na+, 350.0900; found, 350.0916.
1.5). Yield 61 mg (75%); yellow solid; mp 219–221 °C. 1H NMR (500 MHz, DMSO-d6 + CDCl3) δ 12.98 (s, 1H), 8.72 (s, 1H), 7.62–7.57 (m, 3H), 7.49 (t, J = 8.0 Hz, 1H), 7.35 (dd, J = 8.2, 2.7 Hz, 1H), 7.27–7.22 (m, 2H), 7.12 (t, J = 7.7 Hz, 1H), 3.83 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.6, 163.9, 159.9, 137.0, 135.2, 134.5, 130.9, 124.0, 123.8, 123.0, 122.8, 121.9, 119.7, 115.1, 113.9, 113.7, 113.4, 108.1, 69.9, 55.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C20H13N3O2Na+, 350.0900; found, 350.0916.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5). Yield 55 mg (64%); yellow solid; mp 246–248 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.05 (s, 1H), 8.73 (s, 1H), 8.35 (s, 4H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.21 (d, J = 8.1 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.2, 162.5, 151.6, 137.4, 137.2, 135.8, 131.4, 124.9, 124.4, 123.7, 123.4, 119.7, 114.9, 114.0, 113.9, 107.9, 70.5. HRMS (ESI-TOF) m/z: [M] calcd for C19H10N4O3, 342.0747; found, 342.0751.
2.5). Yield 55 mg (64%); yellow solid; mp 246–248 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.05 (s, 1H), 8.73 (s, 1H), 8.35 (s, 4H), 7.59 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.6 Hz, 1H), 7.21 (d, J = 8.1 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.2, 162.5, 151.6, 137.4, 137.2, 135.8, 131.4, 124.9, 124.4, 123.7, 123.4, 119.7, 114.9, 114.0, 113.9, 107.9, 70.5. HRMS (ESI-TOF) m/z: [M] calcd for C19H10N4O3, 342.0747; found, 342.0751.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 70 mg (81%); yellow solid; mp 247–249 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.81 (s, 1H), 8.78 (s, 1H), 8.14 (t, J = 5 Hz, 3H), 8.04 (d, J = 8.2 Hz, 1H), 7.74 (t, J = 6.9 Hz, 1H), 7.64 (t, J = 7.0 Hz, 1H), 7.58 (d, J = 7.8 Hz, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.07 (t, J = 10 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.9, 164.1, 137.0, 136.2, 135.4, 133.6, 132.1, 130.7, 130.3, 130.2, 129.7, 127.9, 127.6, 124.0, 123.9, 123.2, 123.1, 119.7, 115.3, 114.2, 113.8, 108.4, 69.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H14N3O+, 348.1131; found, 348.1148.
2). Yield 70 mg (81%); yellow solid; mp 247–249 °C. 1H NMR (500 MHz, DMSO-d6) δ 13.02 (s, 1H), 8.81 (s, 1H), 8.78 (s, 1H), 8.14 (t, J = 5 Hz, 3H), 8.04 (d, J = 8.2 Hz, 1H), 7.74 (t, J = 6.9 Hz, 1H), 7.64 (t, J = 7.0 Hz, 1H), 7.58 (d, J = 7.8 Hz, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.07 (t, J = 10 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.9, 164.1, 137.0, 136.2, 135.4, 133.6, 132.1, 130.7, 130.3, 130.2, 129.7, 127.9, 127.6, 124.0, 123.9, 123.2, 123.1, 119.7, 115.3, 114.2, 113.8, 108.4, 69.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H14N3O+, 348.1131; found, 348.1148.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 80 mg (85%); yellow solid; mp 160–162 °C. 1H NMR (500 MHz, DMSO-d6 + CDCl3) δ 12.98 (s, 1H), 8.79 (s, 1H), 8.66 (s, 1H), 8.09–7.99 (m, 3H), 7.57 (d, J = 8.0 Hz, 1H), 7.45 (d, J = 2.7 Hz, 1H), 7.27 (dd, J = 9.1, 2.6 Hz, 1H), 7.22 (t, J = 8.5 Hz, 2H), 7.06 (t, J = 7.7 Hz, 1H), 3.91 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 192.4, 164.4, 160.7, 138.4, 137.0, 135.2, 133.4, 131.9, 128.7, 128.4, 127.4, 124.0, 123.0, 120.2, 119.8, 115.4, 114.2, 113.8, 108.4, 106.5, 69.8, 55.7. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H16N3O2+, 378.1237; found, 378.1266.
2). Yield 80 mg (85%); yellow solid; mp 160–162 °C. 1H NMR (500 MHz, DMSO-d6 + CDCl3) δ 12.98 (s, 1H), 8.79 (s, 1H), 8.66 (s, 1H), 8.09–7.99 (m, 3H), 7.57 (d, J = 8.0 Hz, 1H), 7.45 (d, J = 2.7 Hz, 1H), 7.27 (dd, J = 9.1, 2.6 Hz, 1H), 7.22 (t, J = 8.5 Hz, 2H), 7.06 (t, J = 7.7 Hz, 1H), 3.91 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 192.4, 164.4, 160.7, 138.4, 137.0, 135.2, 133.4, 131.9, 128.7, 128.4, 127.4, 124.0, 123.0, 120.2, 119.8, 115.4, 114.2, 113.8, 108.4, 106.5, 69.8, 55.7. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H16N3O2+, 378.1237; found, 378.1266.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). Yield 62 mg (82%); yellow solid; mp 227–230 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.72 (s, 1H), 8.33 (d, J = 4.9 Hz, 1H), 7.99 (d, J = 2.7 Hz, 1H), 7.59 (d, J = 8.1 Hz, 1H), 7.30–7.25 (m, 3H), 7.15 (t, J = 7.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 184.6, 162.8, 140.0, 138.6, 137.0, 135.4, 129.9, 124.0, 123.9, 123.0, 119.6, 115.1, 113.9, 113.7, 108.2, 70.2. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C17H9N3OSNa+, 326.0359; found, 326.0381.
1.5). Yield 62 mg (82%); yellow solid; mp 227–230 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.72 (s, 1H), 8.33 (d, J = 4.9 Hz, 1H), 7.99 (d, J = 2.7 Hz, 1H), 7.59 (d, J = 8.1 Hz, 1H), 7.30–7.25 (m, 3H), 7.15 (t, J = 7.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 184.6, 162.8, 140.0, 138.6, 137.0, 135.4, 129.9, 124.0, 123.9, 123.0, 119.6, 115.1, 113.9, 113.7, 108.2, 70.2. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C17H9N3OSNa+, 326.0359; found, 326.0381.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 65 mg (83%); yellow solid; mp 155–157 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.68 (s, 1H), 8.07 (d, J = 7.6 Hz, 2H), 7.77 (t, J = 7.6 Hz, 1H), 7.59 (t, J = 7.8 Hz, 2H), 7.07–6.99 (m, 3H), 2.49 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.9, 164.1, 136.4, 135.9, 134.6, 133.2, 129.8, 129.7, 124.7, 123.7, 123.25, 123.22, 117.2, 115.2, 108.5, 70.1, 16.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1108.
2). Yield 65 mg (83%); yellow solid; mp 155–157 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.68 (s, 1H), 8.07 (d, J = 7.6 Hz, 2H), 7.77 (t, J = 7.6 Hz, 1H), 7.59 (t, J = 7.8 Hz, 2H), 7.07–6.99 (m, 3H), 2.49 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.9, 164.1, 136.4, 135.9, 134.6, 133.2, 129.8, 129.7, 124.7, 123.7, 123.25, 123.22, 117.2, 115.2, 108.5, 70.1, 16.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1108.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 58 mg (74%); yellow solid; mp 227–229 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.06 (s, 1H), 8.70 (s, 1H), 8.09 (d, J = 7.5 Hz, 2H), 7.79 (t, J = 7.5 Hz, 1H), 7.61 (t, J = 7.0 Hz, 3H), 7.14 (t, J = 7.8 Hz, 1H), 6.96 (d, J = 7.7 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.6, 163.8, 158.6 (d, J = 236 Hz), 136.5, 136.2, 133.8, 133.1, 129.9, 129.8, 124.4 (d, J = 10 Hz), 115.3 (d, J = 10 Hz), 114.8, 113.8, 112.3 (d, J = 26 Hz), 107.9, 105.3 (d, J = 26 Hz), 71.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11FN3O+, 316.0881; found, 316.0865.
2). Yield 58 mg (74%); yellow solid; mp 227–229 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.06 (s, 1H), 8.70 (s, 1H), 8.09 (d, J = 7.5 Hz, 2H), 7.79 (t, J = 7.5 Hz, 1H), 7.61 (t, J = 7.0 Hz, 3H), 7.14 (t, J = 7.8 Hz, 1H), 6.96 (d, J = 7.7 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.6, 163.8, 158.6 (d, J = 236 Hz), 136.5, 136.2, 133.8, 133.1, 129.9, 129.8, 124.4 (d, J = 10 Hz), 115.3 (d, J = 10 Hz), 114.8, 113.8, 112.3 (d, J = 26 Hz), 107.9, 105.3 (d, J = 26 Hz), 71.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11FN3O+, 316.0881; found, 316.0865.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 72 mg (77%); yellow solid; mp 260–262 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.66 (s, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.80 (t, J = 7.5 Hz, 1H), 7.62 (t, J = 7.8 Hz, 2H), 7.55 (d, J = 8.6 Hz, 1H), 7.44 (s, 1H), 7.40 (d, J = 8.6 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.5, 163.6, 136.2, 136.1, 135.9, 133.0, 129.9, 129.8, 126.7, 125.4, 122.3, 115.7, 115.5, 114.7, 113.6, 107.2, 71.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11BrN3O+, 376.0080; found, 376.0097.
2). Yield 72 mg (77%); yellow solid; mp 260–262 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.66 (s, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.80 (t, J = 7.5 Hz, 1H), 7.62 (t, J = 7.8 Hz, 2H), 7.55 (d, J = 8.6 Hz, 1H), 7.44 (s, 1H), 7.40 (d, J = 8.6 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.5, 163.6, 136.2, 136.1, 135.9, 133.0, 129.9, 129.8, 126.7, 125.4, 122.3, 115.7, 115.5, 114.7, 113.6, 107.2, 71.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H11BrN3O+, 376.0080; found, 376.0097.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 68 mg (83%); yellow solid; mp 227–229 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.61 (s, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.79 (t, J = 7.4 Hz, 1H), 7.61 (t, J = 8.4 Hz, 2H), 7.48 (d, J = 8.9 Hz, 1H), 6.89 (dd, J = 8.9, 2.4 Hz, 1H), 6.69 (d, J = 2.4 Hz, 1H), 3.57 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 163.9, 155.8, 136.0, 135.3, 133.2, 131.8, 129.8, 124.6, 115.2, 114.5, 114.2, 113.2, 108.0, 102.8, 68.8, 55.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O2+, 328.1081; found, 328.1088.
2). Yield 68 mg (83%); yellow solid; mp 227–229 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.91 (s, 1H), 8.61 (s, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.79 (t, J = 7.4 Hz, 1H), 7.61 (t, J = 8.4 Hz, 2H), 7.48 (d, J = 8.9 Hz, 1H), 6.89 (dd, J = 8.9, 2.4 Hz, 1H), 6.69 (d, J = 2.4 Hz, 1H), 3.57 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 163.9, 155.8, 136.0, 135.3, 133.2, 131.8, 129.8, 124.6, 115.2, 114.5, 114.2, 113.2, 108.0, 102.8, 68.8, 55.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O2+, 328.1081; found, 328.1088.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 65 mg (84%); yellow solid; mp 190–192 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.77 (t, J = 7.5 Hz, 1H), 7.66 (d, J = 9.2 Hz, 1H), 7.60 (t, J = 7.5 Hz, 2H), 7.33 (t, J = 7.7 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 7.18 (t, J = 7.7 Hz, 1H), 3.99 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 163.4, 138.2, 137.8, 136.1, 133.3, 129.85, 129.82, 124.2, 123.5, 120.1, 115.0, 114.1, 112.4, 107.2, 69.6, 34.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1116.
2). Yield 65 mg (84%); yellow solid; mp 190–192 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.77 (t, J = 7.5 Hz, 1H), 7.66 (d, J = 9.2 Hz, 1H), 7.60 (t, J = 7.5 Hz, 2H), 7.33 (t, J = 7.7 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 7.18 (t, J = 7.7 Hz, 1H), 3.99 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 192.9, 163.4, 138.2, 137.8, 136.1, 133.3, 129.85, 129.82, 124.2, 123.5, 120.1, 115.0, 114.1, 112.4, 107.2, 69.6, 34.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1116.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 75 mg (77%); yellow solid; mp 195–197 °C. 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 8.03 (d, J = 7.2 Hz, 2H), 7.67 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.5 Hz, 2H), 7.40–7.30 (m, 5H), 7.26–7.20 (m, 3H), 7.11 (t, J = 8.27 Hz, 1H), 5.43 (s, 2H). 13C{1H} NMR (125 MHz, CDCl3) δ 192.8, 164.0, 137.2, 135.8, 135.7, 134.3, 134.0, 130.0, 129.6, 129.4, 128.9, 127.4, 125.5, 124.7, 123.9, 121.2, 115.3, 113.2, 111.6, 108.9, 51.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H18N3O+, 388.1444; found, 388.1414.
2). Yield 75 mg (77%); yellow solid; mp 195–197 °C. 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 8.03 (d, J = 7.2 Hz, 2H), 7.67 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.5 Hz, 2H), 7.40–7.30 (m, 5H), 7.26–7.20 (m, 3H), 7.11 (t, J = 8.27 Hz, 1H), 5.43 (s, 2H). 13C{1H} NMR (125 MHz, CDCl3) δ 192.8, 164.0, 137.2, 135.8, 135.7, 134.3, 134.0, 130.0, 129.6, 129.4, 128.9, 127.4, 125.5, 124.7, 123.9, 121.2, 115.3, 113.2, 111.6, 108.9, 51.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H18N3O+, 388.1444; found, 388.1414.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 56 mg (72%); yellow solid; mp 198–200 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.58 (s, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.70 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.8 Hz, 2H), 7.41 (d, J = 8.7 Hz, 2H), 7.22–7.12 (m, 2H), 2.55 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.7, 165.1, 144.3, 136.1, 135.8, 133.6, 129.6, 124.9, 123.5, 122.1, 119.7, 114.2, 113.7, 112.3, 106.9, 77.1, 15.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1137.
2). Yield 56 mg (72%); yellow solid; mp 198–200 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.58 (s, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.70 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.8 Hz, 2H), 7.41 (d, J = 8.7 Hz, 2H), 7.22–7.12 (m, 2H), 2.55 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 192.7, 165.1, 144.3, 136.1, 135.8, 133.6, 129.6, 124.9, 123.5, 122.1, 119.7, 114.2, 113.7, 112.3, 106.9, 77.1, 15.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H14N3O+, 312.1131; found, 312.1137.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 65 mg (70%); red solid; mp 192–194 °C. 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H), 7.65–7.63 (m, 1H), 7.48–7.43 (m, 3H), 7.40–7.33 (m, 6H), 7.24–7.20 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3) δ 190.9, 164.6, 144.6, 136.2, 134.8, 134.5, 130.6, 129.9, 129.4, 129.31, 129.29, 128.7, 125.3, 124.8, 122.9, 120.9, 113.7, 112.9, 112.3, 108.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H16N3O+, 374.1288; found, 374.1298.
2). Yield 65 mg (70%); red solid; mp 192–194 °C. 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H), 7.65–7.63 (m, 1H), 7.48–7.43 (m, 3H), 7.40–7.33 (m, 6H), 7.24–7.20 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3) δ 190.9, 164.6, 144.6, 136.2, 134.8, 134.5, 130.6, 129.9, 129.4, 129.31, 129.29, 128.7, 125.3, 124.8, 122.9, 120.9, 113.7, 112.9, 112.3, 108.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H16N3O+, 374.1288; found, 374.1298.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 63 mg (67%); yellow solid; mp 218–220 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.71 (s, 1H), 8.67 (s, 1H), 7.96 (d, J = 7.2 Hz, 2H), 7.87 (d, J = 7.1 Hz, 2H), 7.66 (t, J = 7.5 Hz, 1H), 7.59–7.52 (m, 4H), 7.46 (t, J = 7.8 Hz, 2H), 7.41 (d, J = 8.1 Hz, 1H), 7.22 (t, J = 7.2 Hz, 1H), 7.10 (t, J = 7.3 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.8, 187.9, 162.4, 137.1, 136.4, 135.4, 134.4, 133.8, 133.2, 128.9, 128.6, 128.5, 124.2, 123.5, 122.2, 120.6, 119.5, 113.4, 109.3, 101.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H17N2O2+, 377.1285; found, 377.1301.
2). Yield 63 mg (67%); yellow solid; mp 218–220 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.71 (s, 1H), 8.67 (s, 1H), 7.96 (d, J = 7.2 Hz, 2H), 7.87 (d, J = 7.1 Hz, 2H), 7.66 (t, J = 7.5 Hz, 1H), 7.59–7.52 (m, 4H), 7.46 (t, J = 7.8 Hz, 2H), 7.41 (d, J = 8.1 Hz, 1H), 7.22 (t, J = 7.2 Hz, 1H), 7.10 (t, J = 7.3 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.8, 187.9, 162.4, 137.1, 136.4, 135.4, 134.4, 133.8, 133.2, 128.9, 128.6, 128.5, 124.2, 123.5, 122.2, 120.6, 119.5, 113.4, 109.3, 101.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H17N2O2+, 377.1285; found, 377.1301.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Yield 66 mg (68%); yellow solid; mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.70 (s, 1H), 8.65 (s, 1H), 7.95 (d, J = 7.1 Hz, 2H), 7.79 (d, J = 8.2 Hz, 2H), 7.59–7.53 (m, 2H), 7.45 (t, J = 7.7 Hz, 2H), 7.40 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.2 Hz, 2H), 7.22 (t, J = 7.0 Hz, 1H), 7.09 (t, J = 7.7 Hz, 1H), 2.38 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 193.9, 187.5, 162.2, 143.9, 137.1, 135.4, 134.1, 133.8, 133.6, 129.1, 128.97, 128.94, 128.6, 124.2, 123.5, 122.2, 120.6, 119.6, 113.4, 109.3, 101.9, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H19N2O2+, 391.1441; found, 391.1465.
2). Yield 66 mg (68%); yellow solid; mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.70 (s, 1H), 8.65 (s, 1H), 7.95 (d, J = 7.1 Hz, 2H), 7.79 (d, J = 8.2 Hz, 2H), 7.59–7.53 (m, 2H), 7.45 (t, J = 7.7 Hz, 2H), 7.40 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.2 Hz, 2H), 7.22 (t, J = 7.0 Hz, 1H), 7.09 (t, J = 7.7 Hz, 1H), 2.38 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 193.9, 187.5, 162.2, 143.9, 137.1, 135.4, 134.1, 133.8, 133.6, 129.1, 128.97, 128.94, 128.6, 124.2, 123.5, 122.2, 120.6, 119.6, 113.4, 109.3, 101.9, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H19N2O2+, 391.1441; found, 391.1465.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 28 mg (89%); pale yellow solid; mp 344–346 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.32 (s, 1H), 8.05 (d, J = 8.1 Hz, 1H), 7.99 (d, J = 3.2 Hz, 1H), 7.58 (s, 1H), 7.46 (t, J = 8.6 Hz, 3H), 7.30 (t, J = 7.6 Hz, 2H), 7.24–7.13 (m, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.5, 166.4, 141.0, 136.6, 132.7, 128.4, 128.1, 125.3, 124.7, 123.0, 121.9, 121.1, 115.9, 112.7, 106.5, 96.9, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H14N3O2+, 316.1081; found, 316.1084.
3). Yield 28 mg (89%); pale yellow solid; mp 344–346 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.32 (s, 1H), 8.05 (d, J = 8.1 Hz, 1H), 7.99 (d, J = 3.2 Hz, 1H), 7.58 (s, 1H), 7.46 (t, J = 8.6 Hz, 3H), 7.30 (t, J = 7.6 Hz, 2H), 7.24–7.13 (m, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.5, 166.4, 141.0, 136.6, 132.7, 128.4, 128.1, 125.3, 124.7, 123.0, 121.9, 121.1, 115.9, 112.7, 106.5, 96.9, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H14N3O2+, 316.1081; found, 316.1084.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 30 mg (87%); pale yellow solid; mp 248–250 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.26 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H), 8.00 (d, J = 3.3 Hz, 1H), 7.51 (s, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.22–7.12 (m, 4H), 2.54–2.48 (m, 2H), 1.09 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.6, 166.4, 143.6, 138.4, 136.5, 132.7, 127.8, 125.3, 124.8, 122.9, 121.9, 121.1, 115.9, 112.7, 106.5, 96.8, 89.5, 27.7, 15.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H18N3O2+, 344.1394; found, 344.1396.
3). Yield 30 mg (87%); pale yellow solid; mp 248–250 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.26 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H), 8.00 (d, J = 3.3 Hz, 1H), 7.51 (s, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.22–7.12 (m, 4H), 2.54–2.48 (m, 2H), 1.09 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.6, 166.4, 143.6, 138.4, 136.5, 132.7, 127.8, 125.3, 124.8, 122.9, 121.9, 121.1, 115.9, 112.7, 106.5, 96.8, 89.5, 27.7, 15.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H18N3O2+, 344.1394; found, 344.1396.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 28 mg (80%); yellow solid; mp 216–218 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.18 (s, 1H), 9.34 (s, 1H), 8.05 (d, J = 7.9 Hz, 1H), 8.01 (d, J = 3.2 Hz, 1H), 7.69 (s, 1H), 7.48 (t, J = 9.2 Hz, 3H), 7.35 (d, J = 8.6 Hz, 2H), 7.18 (dt, J = 21.3, 6.8 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.3, 166.0, 140.1, 136.6, 132.74, 132.72, 128.4, 127.4, 124.7, 123.1, 121.9, 121.2, 115.8, 112.8, 106.4, 96.9, 89.1. HRMS (ESI-TOF) m/z: [M + H+] calcd for C19H13ClN3O2+, 350.0691; found, 350.0702.
3). Yield 28 mg (80%); yellow solid; mp 216–218 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.18 (s, 1H), 9.34 (s, 1H), 8.05 (d, J = 7.9 Hz, 1H), 8.01 (d, J = 3.2 Hz, 1H), 7.69 (s, 1H), 7.48 (t, J = 9.2 Hz, 3H), 7.35 (d, J = 8.6 Hz, 2H), 7.18 (dt, J = 21.3, 6.8 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.3, 166.0, 140.1, 136.6, 132.74, 132.72, 128.4, 127.4, 124.7, 123.1, 121.9, 121.2, 115.8, 112.8, 106.4, 96.9, 89.1. HRMS (ESI-TOF) m/z: [M + H+] calcd for C19H13ClN3O2+, 350.0691; found, 350.0702.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 36 mg (82%); light brown solid; charred at 350 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.20 (s, 1H), 9.33 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 8.01 (s, 1H), 7.67 (d, J = 4.6 Hz, 2H), 7.64 (s, 1H), 7.46 (d, J = 7.8 Hz, 1H), 7.27 (d, J = 8.6 Hz, 2H), 7.18 (dt, J = 21.5, 7.0 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.3, 165.9, 140.9, 137.2, 136.6, 132.7, 127.7, 124.6, 123.1, 121.9, 121.2, 112.8, 106.4, 96.9, 94.6, 89.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13IN3O2+, 442.0047; found, 442.0042.
3). Yield 36 mg (82%); light brown solid; charred at 350 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.20 (s, 1H), 9.33 (s, 1H), 8.04 (d, J = 8.9 Hz, 1H), 8.01 (s, 1H), 7.67 (d, J = 4.6 Hz, 2H), 7.64 (s, 1H), 7.46 (d, J = 7.8 Hz, 1H), 7.27 (d, J = 8.6 Hz, 2H), 7.18 (dt, J = 21.5, 7.0 Hz, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.3, 165.9, 140.9, 137.2, 136.6, 132.7, 127.7, 124.6, 123.1, 121.9, 121.2, 112.8, 106.4, 96.9, 94.6, 89.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13IN3O2+, 442.0047; found, 442.0042.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 29 mg (84%); orange solid; mp 226–228 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.25 (s, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.98 (d, J = 3.2 Hz, 1H), 7.49 (s, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.37 (d, J = 8.9 Hz, 2H), 7.22–7.13 (m, 2H), 6.84 (d, J = 8.9 Hz, 2H), 3.67 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.7, 166.3, 158.9, 136.6, 132.9, 132.6, 126.7, 124.8, 122.9, 121.9, 121.1, 115.9, 113.7, 112.7, 106.5, 96.8, 89.5, 55.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O3+, 346.1186; found, 346.1204.
3). Yield 29 mg (84%); orange solid; mp 226–228 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 9.25 (s, 1H), 8.03 (d, J = 7.8 Hz, 1H), 7.98 (d, J = 3.2 Hz, 1H), 7.49 (s, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.37 (d, J = 8.9 Hz, 2H), 7.22–7.13 (m, 2H), 6.84 (d, J = 8.9 Hz, 2H), 3.67 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.7, 166.3, 158.9, 136.6, 132.9, 132.6, 126.7, 124.8, 122.9, 121.9, 121.1, 115.9, 113.7, 112.7, 106.5, 96.8, 89.5, 55.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O3+, 346.1186; found, 346.1204.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 29 mg (80%); yellow solid; mp 232–235 °C. 1H NMR (400 MHz, DMSO-d6 + CDCl3) δ 12.21 (s, 1H), 9.47 (s, 1H), 8.15 (d, J = 9.0 Hz, 2H), 8.06 (d, J = 7.1 Hz, 2H), 7.94 (s, 1H), 7.77 (d, J = 9.0 Hz, 2H), 7.46 (d, J = 7.1 Hz, 1H), 7.22–7.14 (m, 2H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 166.4, 165.3, 148.1, 147.1, 136.6, 132.7, 126.8, 124.5, 123.5, 122.9, 121.9, 121.1, 115.6, 112.6, 106.2, 97.2, 88.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13N4O4+, 361.0931; found, 361.0901.
3). Yield 29 mg (80%); yellow solid; mp 232–235 °C. 1H NMR (400 MHz, DMSO-d6 + CDCl3) δ 12.21 (s, 1H), 9.47 (s, 1H), 8.15 (d, J = 9.0 Hz, 2H), 8.06 (d, J = 7.1 Hz, 2H), 7.94 (s, 1H), 7.77 (d, J = 9.0 Hz, 2H), 7.46 (d, J = 7.1 Hz, 1H), 7.22–7.14 (m, 2H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 166.4, 165.3, 148.1, 147.1, 136.6, 132.7, 126.8, 124.5, 123.5, 122.9, 121.9, 121.1, 115.6, 112.6, 106.2, 97.2, 88.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13N4O4+, 361.0931; found, 361.0901.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 29 mg (88%); light brown solid; mp 261–263 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.32 (s, 1H), 8.10 (s, 1H), 8.03 (d, J = 7.9 Hz, 1H), 7.61 (s, 1H), 7.49 (t, J = 7.5 Hz, 3H), 7.28 (q, J = 8.4, 7.8 Hz, 3H), 7.21 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.4, 166.1, 140.9, 137.1, 135.8, 128.4, 128.1, 125.3, 125.1, 123.0, 122.1, 121.4, 115.9, 111.2, 105.5, 96.8, 89.6, 33.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O2+, 330.1237; found, 330.1243.
3). Yield 29 mg (88%); light brown solid; mp 261–263 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.32 (s, 1H), 8.10 (s, 1H), 8.03 (d, J = 7.9 Hz, 1H), 7.61 (s, 1H), 7.49 (t, J = 7.5 Hz, 3H), 7.28 (q, J = 8.4, 7.8 Hz, 3H), 7.21 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.4, 166.1, 140.9, 137.1, 135.8, 128.4, 128.1, 125.3, 125.1, 123.0, 122.1, 121.4, 115.9, 111.2, 105.5, 96.8, 89.6, 33.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O2+, 330.1237; found, 330.1243.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 33 mg (81%); pale yellow solid; mp 224–226 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.35 (s, 1H), 8.29 (s, 1H), 8.02 (d, J = 7.9 Hz, 1H), 7.61 (s, 1H), 7.53 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.30–7.16 (m, 8H), 7.08 (d, J = 7.5 Hz, 2H), 5.48 (s, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.4, 165.9, 140.9, 136.6, 136.2, 135.4, 128.6, 128.3, 128.0, 127.8, 127.3, 125.5, 125.4, 123.0, 121.9, 121.4, 115.7, 111.5, 105.7, 97.3, 89.6, 49.5. HRMS (ESI-TOF) m/z: [M + H+] calcd for C26H20N3O2+, 406.1550; found, 406.1578.
3). Yield 33 mg (81%); pale yellow solid; mp 224–226 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.35 (s, 1H), 8.29 (s, 1H), 8.02 (d, J = 7.9 Hz, 1H), 7.61 (s, 1H), 7.53 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.30–7.16 (m, 8H), 7.08 (d, J = 7.5 Hz, 2H), 5.48 (s, 2H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.4, 165.9, 140.9, 136.6, 136.2, 135.4, 128.6, 128.3, 128.0, 127.8, 127.3, 125.5, 125.4, 123.0, 121.9, 121.4, 115.7, 111.5, 105.7, 97.3, 89.6, 49.5. HRMS (ESI-TOF) m/z: [M + H+] calcd for C26H20N3O2+, 406.1550; found, 406.1578.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 28 mg (85%); yellow solid; mp 275–277 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.17 (s, 1H), 9.32 (s, 1H), 7.93 (d, J = 3.3 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.59 (s, 1H), 7.47 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 7.5 Hz, 2H), 7.22 (t, J = 7.3 Hz, 1H), 7.06 (t, J = 7.6 Hz, 1H), 7.00 (d, J = 7.2 Hz, 1H), 2.42 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.6, 166.4, 141.1, 135.9, 132.1, 128.4, 128.1, 125.3, 124.6, 123.6, 121.9, 121.3, 119.5, 115.9, 106.9, 97.0, 89.6, 16.7. HRMS (ESI-TOF) m/z: [M + H+] calcd for C20H16N3O2+, 330.1237; found, 330.1262.
3). Yield 28 mg (85%); yellow solid; mp 275–277 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.17 (s, 1H), 9.32 (s, 1H), 7.93 (d, J = 3.3 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.59 (s, 1H), 7.47 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 7.5 Hz, 2H), 7.22 (t, J = 7.3 Hz, 1H), 7.06 (t, J = 7.6 Hz, 1H), 7.00 (d, J = 7.2 Hz, 1H), 2.42 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.6, 166.4, 141.1, 135.9, 132.1, 128.4, 128.1, 125.3, 124.6, 123.6, 121.9, 121.3, 119.5, 115.9, 106.9, 97.0, 89.6, 16.7. HRMS (ESI-TOF) m/z: [M + H+] calcd for C20H16N3O2+, 330.1237; found, 330.1262.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 29 mg (87%); pale yellow solid; mp 232–234 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.34 (s, 1H), 9.31 (s, 1H), 8.10 (s, 1H), 7.78 (d, J = 10.9 Hz, 1H), 7.61 (s, 1H), 7.47 (t, J = 7.3 Hz, 3H), 7.30 (t, J = 7.5 Hz, 2H), 7.23 (t, J = 7.3 Hz, 1H), 7.06 (t, J = 9.0 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 166.4, 166.2, 158.4, 158.2, 157.8 (d, J = 232.5 Hz), 140.8, 134.4, 133.4, 128.5, 128.3, 125.3 (d, J = 17.5 Hz), 118.6, 116.2, 116.0, 114.0 (d, J = 10 Hz), 111.2 (d, J = 26.25 Hz), 107.4 (d, J = 25 Hz), 106.8, 97.0, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13FN3O2+, 334.0986; found, 334.1000.
3). Yield 29 mg (87%); pale yellow solid; mp 232–234 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.34 (s, 1H), 9.31 (s, 1H), 8.10 (s, 1H), 7.78 (d, J = 10.9 Hz, 1H), 7.61 (s, 1H), 7.47 (t, J = 7.3 Hz, 3H), 7.30 (t, J = 7.5 Hz, 2H), 7.23 (t, J = 7.3 Hz, 1H), 7.06 (t, J = 9.0 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 166.4, 166.2, 158.4, 158.2, 157.8 (d, J = 232.5 Hz), 140.8, 134.4, 133.4, 128.5, 128.3, 125.3 (d, J = 17.5 Hz), 118.6, 116.2, 116.0, 114.0 (d, J = 10 Hz), 111.2 (d, J = 26.25 Hz), 107.4 (d, J = 25 Hz), 106.8, 97.0, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13FN3O2+, 334.0986; found, 334.1000.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 28 mg (80%); yellow solid; mp 186–188 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.20 (s, 1H), 9.36 (s, 1H), 8.04 (s, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.60 (s, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.47 (d, J = 7.0 Hz, 2H), 7.30 (t, J = 7.4 Hz, 2H), 7.23 (t, J = 7.2 Hz, 1H), 7.17 (dd, J = 8.7, 2.0 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.2, 166.1, 140.6, 137.0, 133.4, 128.4, 128.2, 127.6, 125.3, 123.4, 123.3, 121.3, 115.7, 112.4, 106.5, 97.9, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13ClN3O2+, 350.0691; found, 350.0689.
3). Yield 28 mg (80%); yellow solid; mp 186–188 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.20 (s, 1H), 9.36 (s, 1H), 8.04 (s, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.60 (s, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.47 (d, J = 7.0 Hz, 2H), 7.30 (t, J = 7.4 Hz, 2H), 7.23 (t, J = 7.2 Hz, 1H), 7.17 (dd, J = 8.7, 2.0 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.2, 166.1, 140.6, 137.0, 133.4, 128.4, 128.2, 127.6, 125.3, 123.4, 123.3, 121.3, 115.7, 112.4, 106.5, 97.9, 89.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13ClN3O2+, 350.0691; found, 350.0689.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 34 mg (86%); yellow solid; mp 270–272 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H), 9.34 (s, 1H), 8.22 (d, J = 2.0 Hz, 1H), 8.07 (s, 1H), 7.60 (s, 1H), 7.48 (d, J = 7.1 Hz, 2H), 7.42 (d, J = 8.6 Hz, 1H), 7.30 (t, J = 7.8 Hz, 3H), 7.24 (t, J = 7.2 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.1, 165.9, 140.5, 135.3, 133.6, 128.4, 128.2, 126.2, 125.5, 125.3, 124.4, 115.8, 114.6, 113.7, 106.0, 97.7, 89.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13N3O2Br+, 394.0186; found, 394.0199.
3). Yield 34 mg (86%); yellow solid; mp 270–272 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H), 9.34 (s, 1H), 8.22 (d, J = 2.0 Hz, 1H), 8.07 (s, 1H), 7.60 (s, 1H), 7.48 (d, J = 7.1 Hz, 2H), 7.42 (d, J = 8.6 Hz, 1H), 7.30 (t, J = 7.8 Hz, 3H), 7.24 (t, J = 7.2 Hz, 1H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 166.1, 165.9, 140.5, 135.3, 133.6, 128.4, 128.2, 126.2, 125.5, 125.3, 124.4, 115.8, 114.6, 113.7, 106.0, 97.7, 89.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H13N3O2Br+, 394.0186; found, 394.0199.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield 29 mg (84%); pale yellow solid; mp 230–232 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 9.25 (s, 1H), 7.97 (d, J = 3.3 Hz, 1H), 7.55 (d, J = 10.5 Hz, 2H), 7.48 (d, J = 7.9 Hz, 2H), 7.35–7.29 (m, 3H), 7.24 (t, J = 7.2 Hz, 1H), 6.82 (d, J = 8.8 Hz, 1H), 3.74 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 166.4, 166.3, 154.7, 141.2, 133.1, 131.3, 128.4, 128.0, 125.4, 125.2, 116.2, 113.3, 112.8, 106.6, 104.3, 95.7, 89.4, 55.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O3+, 346.1186; found, 346.1162.
3). Yield 29 mg (84%); pale yellow solid; mp 230–232 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 9.25 (s, 1H), 7.97 (d, J = 3.3 Hz, 1H), 7.55 (d, J = 10.5 Hz, 2H), 7.48 (d, J = 7.9 Hz, 2H), 7.35–7.29 (m, 3H), 7.24 (t, J = 7.2 Hz, 1H), 6.82 (d, J = 8.8 Hz, 1H), 3.74 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 166.4, 166.3, 154.7, 141.2, 133.1, 131.3, 128.4, 128.0, 125.4, 125.2, 116.2, 113.3, 112.8, 106.6, 104.3, 95.7, 89.4, 55.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16N3O3+, 346.1186; found, 346.1162.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 19 mg (76%); pale yellow solid; mp 191–193 °C. 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.48 (d, J = 8.8 Hz, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.91 (d, J = 3.3 Hz, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.52–7.45 (m, 3H), 7.40–7.34 (m, 2H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 193.9, 188.4, 137.0, 136.5, 134.2, 133.5, 130.2, 128.7, 125.6, 123.9, 122.9, 122.1, 113.7, 112.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H12NO2+, 250.0863; found, 250.0872.
1). Yield 19 mg (76%); pale yellow solid; mp 191–193 °C. 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.48 (d, J = 8.8 Hz, 1H), 8.09 (d, J = 7.1 Hz, 2H), 7.91 (d, J = 3.3 Hz, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.52–7.45 (m, 3H), 7.40–7.34 (m, 2H). 13C{1H} NMR (125 MHz, DMSO-d6 + CDCl3) δ 193.9, 188.4, 137.0, 136.5, 134.2, 133.5, 130.2, 128.7, 125.6, 123.9, 122.9, 122.1, 113.7, 112.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H12NO2+, 250.0863; found, 250.0872.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 22 mg (79%); pale yellow solid; mp 147–149 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.40 (s, 1H), 8.22–8.19 (m, 1H), 8.15 (s, 1H), 7.88 (d, J = 8.3 Hz, 2H), 7.56–7.54 (m, 1H), 7.43 (d, J = 8.3 Hz, 2H), 7.33–7.28 (m, 2H), 2.70 (q, J = 7.6 Hz, 2H), 1.20 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 193.7, 188.9, 151.5, 137.8, 136.9, 130.8, 129.9, 128.6, 125.0, 123.8, 122.8, 121.2, 112.8, 112.6, 28.4, 15.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H16NO2+, 278.1176; found, 278.1146.
1). Yield 22 mg (79%); pale yellow solid; mp 147–149 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.40 (s, 1H), 8.22–8.19 (m, 1H), 8.15 (s, 1H), 7.88 (d, J = 8.3 Hz, 2H), 7.56–7.54 (m, 1H), 7.43 (d, J = 8.3 Hz, 2H), 7.33–7.28 (m, 2H), 2.70 (q, J = 7.6 Hz, 2H), 1.20 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6) δ 193.7, 188.9, 151.5, 137.8, 136.9, 130.8, 129.9, 128.6, 125.0, 123.8, 122.8, 121.2, 112.8, 112.6, 28.4, 15.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H16NO2+, 278.1176; found, 278.1146.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 19 mg (72%); pale yellow solid; mp 191–193 °C. 1H NMR (400 MHz, CDCl3) δ 8.49–8.46 (m, 1H), 8.10 (d, J = 7.0 Hz, 2H), 7.81 (s, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 7.41–7.39 (m, 3H), 3.84 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 193.9, 187.7, 139.7, 137.9, 134.4, 133.6, 130.5, 128.9, 126.5, 124.4, 123.6, 122.8, 113.0, 110.1, 33.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H14NO2+, 264.1019; found, 264.1018.
1). Yield 19 mg (72%); pale yellow solid; mp 191–193 °C. 1H NMR (400 MHz, CDCl3) δ 8.49–8.46 (m, 1H), 8.10 (d, J = 7.0 Hz, 2H), 7.81 (s, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 7.41–7.39 (m, 3H), 3.84 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 193.9, 187.7, 139.7, 137.9, 134.4, 133.6, 130.5, 128.9, 126.5, 124.4, 123.6, 122.8, 113.0, 110.1, 33.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H14NO2+, 264.1019; found, 264.1018.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 19 mg (71%); white solid; mp 194–195 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.52 (s, 1H), 8.25 (s, 1H), 7.97 (d, J = 7.0 Hz, 2H), 7.89 (dd, J = 9.5, 2.6 Hz, 1H), 7.75 (t, J = 7.4 Hz, 1H), 7.62–7.56 (m, 3H), 7.18 (td, J = 9.2, 2.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.7, 188.3, 159.1 (d, J = 235 Hz), 139.3, 134.8, 133.6, 132.8, 129.8, 129.2, 125.7 (d, J = 11.25 Hz), 114.2 (d, J = 10 Hz), 112.64, 112.61, 112.0 (d, J = 25 Hz), 106.3, 106.2 (d, J = 25). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H11FNO2+, 268.0768; found, 268.0748.
1). Yield 19 mg (71%); white solid; mp 194–195 °C. 1H NMR (500 MHz, DMSO-d6) δ 12.52 (s, 1H), 8.25 (s, 1H), 7.97 (d, J = 7.0 Hz, 2H), 7.89 (dd, J = 9.5, 2.6 Hz, 1H), 7.75 (t, J = 7.4 Hz, 1H), 7.62–7.56 (m, 3H), 7.18 (td, J = 9.2, 2.7 Hz, 1H). 13C{1H} NMR (125 MHz, DMSO-d6) δ 193.7, 188.3, 159.1 (d, J = 235 Hz), 139.3, 134.8, 133.6, 132.8, 129.8, 129.2, 125.7 (d, J = 11.25 Hz), 114.2 (d, J = 10 Hz), 112.64, 112.61, 112.0 (d, J = 25 Hz), 106.3, 106.2 (d, J = 25). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H11FNO2+, 268.0768; found, 268.0748.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 26 mg (81%); pale yellow solid; mp 195–197 °C. 1H NMR (400 MHz, CDCl3) δ 8.56–8.53 (m, 1H), 8.28 (s, 1H), 8.19 (d, J = 8.2 Hz, 1H), 8.11 (d, J = 6.5 Hz, 1H), 7.73 (dt, J = 23.4, 6.8 Hz, 2H), 7.64–7.62 (m, 2H), 7.44–7.39 (m, 3H), 7.36–7.33 (m, 1H), 7.29–7.27 (m, 2H), 6.73 (d, J = 2.9 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 153.9, 149.6, 141.7, 140.4, 139.8, 136.1, 129.9, 129.4, 129.2, 128.99, 128.91, 128.7, 127.9, 126.7, 123.3, 122.3, 121.5, 114.9, 111.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H16N3+, 322.1339; found, 322.1346.
1). Yield 26 mg (81%); pale yellow solid; mp 195–197 °C. 1H NMR (400 MHz, CDCl3) δ 8.56–8.53 (m, 1H), 8.28 (s, 1H), 8.19 (d, J = 8.2 Hz, 1H), 8.11 (d, J = 6.5 Hz, 1H), 7.73 (dt, J = 23.4, 6.8 Hz, 2H), 7.64–7.62 (m, 2H), 7.44–7.39 (m, 3H), 7.36–7.33 (m, 1H), 7.29–7.27 (m, 2H), 6.73 (d, J = 2.9 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 153.9, 149.6, 141.7, 140.4, 139.8, 136.1, 129.9, 129.4, 129.2, 128.99, 128.91, 128.7, 127.9, 126.7, 123.3, 122.3, 121.5, 114.9, 111.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H16N3+, 322.1339; found, 322.1346.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 29 mg (86%); pale yellow solid; mp 193–195 °C. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 7.1 Hz, 1H), 8.17 (d, J = 8.3 Hz, 1H), 8.10 (d, J = 8.2 Hz, 1H), 7.74 (t, J = 7.6 Hz, 1H), 7.69 (d, J = 8.3 Hz, 1H), 7.64 (d, J = 9.5 Hz, 2H), 7.47–7.41 (m, 3H), 7.31–7.27 (m, 2H), 6.60 (s, 1H), 3.64 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 153.8, 149.6, 141.8, 140.5, 139.7, 137.2, 132.4, 129.8, 129.4, 129.1, 129.0, 128.8, 128.7, 127.4, 122.8, 122.6, 121.3, 113.5, 109.4, 33.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H18N3+, 336.1495; found, 336.1488.
1). Yield 29 mg (86%); pale yellow solid; mp 193–195 °C. 1H NMR (400 MHz, CDCl3) δ 8.51 (d, J = 7.1 Hz, 1H), 8.17 (d, J = 8.3 Hz, 1H), 8.10 (d, J = 8.2 Hz, 1H), 7.74 (t, J = 7.6 Hz, 1H), 7.69 (d, J = 8.3 Hz, 1H), 7.64 (d, J = 9.5 Hz, 2H), 7.47–7.41 (m, 3H), 7.31–7.27 (m, 2H), 6.60 (s, 1H), 3.64 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 153.8, 149.6, 141.8, 140.5, 139.7, 137.2, 132.4, 129.8, 129.4, 129.1, 129.0, 128.8, 128.7, 127.4, 122.8, 122.6, 121.3, 113.5, 109.4, 33.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H18N3+, 336.1495; found, 336.1488.
Author contributions
L. H. C. and N. M. conceived and designed the experiments. N. M., V. K., and D. A. performed the experiments, analyzed the data, and prepared the figures. N. M. drafted the manuscript. L. H. C. acquired funding, supervised the laboratory work, revised the manuscript, and prepared the final version.Data availability
The data underlying this study are available in the published article and its online ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank SERB, DST Govt. of India for the financial assistance with sanction no CRG/2021/003716 for this work. Nurabul Mondal thanks the PMRF scheme, Govt. of India for his fellowship. The authors are also grateful to IIT Patna for the basic research facilities. We also acknowledge SAIF IIT Patna for its analytical facilities. We thank IIT Ropar and SAIF IIT Madras for the single crystal XRD facility and BHU for offering the HRMS facility.References
- T. Liu, J. P. Wan and Y. Liu, Chem. Commun., 2021, 57, 9112–9115 RSC.
- (a) Y. Matsuda, H. Gotou, K. Katou, H. Matsumoto, M. Yamashita and S. Maeda, Dyes Pigm., 1990, 14, 225–238 CrossRef CAS; (b) F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902–7917 CrossRef CAS PubMed.
- J. Rosentha, J. Cardiovasc. Pharmacol., 1994, 24, S92–107 CrossRef.
- (a) Z. Hosseinzadeh, A. Ramazani, H. Ahankar, K. Ślepokura and T. Lis, Silicon, 2019, 11, 2933–2943 CrossRef CAS; (b) Z. Hosseinzadeh, A. Ramazani, K. Hosseinzadeh, N. Razzaghi-Asl and F. Gouranlou, Curr. Org. Synth., 2017, 14, 1–13 CrossRef.
- M. S. Abdelbaset, M. Abdel-Aziz, G. E. A. Abuo-Rahma, M. Ramadan and M. H. Abdelrahman, J. Adv. Biomed. Pharm. Sci., 2018, 2, 19–28 Search PubMed.
- (a) A. Dorababu, RSC Med. Chem., 2020, 11, 1335–1353 RSC; (b) W. J. Houlihan, W. A. Remers and R. K. Brown, Indoles: Part I, Wiley, New York, NY, 1992 Search PubMed.
- (a) P. J. Steel, Adv. Heterocycl. Chem., 1996, 67, 1–117 CrossRef CAS; (b) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef CAS PubMed.
- (a) M. Mori, C. Tintori, R. S. A. Christopher, M. Radi, S. Schenone, F. Musumeci, C. Brullo, P. Sanità, S. D. Monache, A. Angelucci and M. Kissova, ChemMedChem, 2013, 8, 484–496 CrossRef CAS PubMed; (b) S. Olla, F. Manetti, E. Crespan, G. Maga, A. Angelucci, S. Schenone, M. Bologna and M. Botta, Bioorg. Med. Chem. Lett., 2009, 19, 1512–1516 CrossRef CAS PubMed.
- A. López-Pérez, S. Freischem, I. Grimm, O. Weiergräber, A. J. Dingley, M. P. López-Alberca, H. Waldmann, W. Vollmer, K. Kumar and C. Vuong, Antibiotics, 2021, 10, 529 CrossRef PubMed.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- (a) R. Banerjee, D. Ali, N. Mondal and L. H. Choudhury, J. Org. Chem., 2024, 89, 4423–4437 CrossRef CAS; (b) P. Bhaumick, N. Mondal and L. H. Choudhury, Eur. J. Org. Chem., 2024, 27, e202400972 CrossRef CAS; (c) P. Bhaumick, R. Kumar, S. S. Acharya, T. Parvin and L. H. Choudhury, J. Org. Chem., 2022, 87, 11399–11413 CrossRef CAS PubMed; (d) A. Jana, D. Ali, P. Bhaumick and L. H. Choudhury, J. Org. Chem., 2022, 87, 7763–7777 CrossRef CAS PubMed.
- T. Arai, Y. Suemitsu and Y. Ikematsu, Org. Lett., 2009, 11, 333–335 CrossRef CAS PubMed.
- H. Lu and Z. Li, Adv. Synth. Catal., 2019, 361, 4474–4482 CrossRef CAS.
- T. Liu, J. P. Wan and Y. Liu, Chem. Commun., 2021, 57, 9112–9115 RSC.
- V. Bhajammanavar, S. Mallik and M. Baidya, Org. Biomol. Chem., 2019, 17, 1740–1743 RSC.
- Y. Huang, Z. Zha and Z. Wang, Org. Lett., 2020, 22, 2512–2516 CrossRef CAS PubMed.
- C. Sujatha, M. Nallagangula and K. Namitharan, Org. Lett., 2021, 23, 4219–4223 CrossRef CAS PubMed.
- J. K. Mansaray, Y. Huang, K. Li, X. Sun, Z. Zha and Z. Wang, Org. Biomol. Chem., 2022, 20, 5510–5514 RSC.
- (a) M. M. Heravi, M. Ghavidel and L. Mohammadkhani, RSC Adv., 2018, 8, 27832–27862 RSC; (b) J. Muzart, Tetrahedron, 2009, 65, 8313–8323 CrossRef CAS.
- (a) D. Wang, Z. Y. Tian, Z. H. Zheng, W. Li, L. N. Wu, J. J. Kuang and J. Z. Yang, Combust. Flame, 2024, 260, 113–240 Search PubMed; (b) L. Čechová, P. Jansa, M. Šála, M. Dračínský, A. Holý and Z. Janeba, Tetrahedron, 2011, 67, 866–871 CrossRef.
- H. T. Nguyen, T. Van Le, P. N. Nguyen, K. H. Nguyen, L. H. T. Nguyen, T. L. H. Doan and P. H. Tran, New J. Chem., 2023, 47, 14733–14745 RSC.
- J. N. Moorthy and N. Singhal, J. Org. Chem., 2005, 70, 1926–1929 CrossRef CAS PubMed.
- A. Suárez, F. Martínez, S. Suárez-Pantiga and R. Sanz, ChemistrySelect, 2017, 2, 787–790 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2376014, 2354497 and 2361275. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00328h |
| This journal is © The Royal Society of Chemistry 2025 |