Multifunctional spinel MnCo2O4 based materials for energy storage and conversion: a review on emerging trends, recent developments and future perspectives
Josué M.
Gonçalves
 *a,
Murillo N. T.
Silva
b,
Kusha Kumar
Naik
c,
Paulo R.
Martins
e,
Diego P.
Rocha
*a,
Murillo N. T.
Silva
b,
Kusha Kumar
Naik
c,
Paulo R.
Martins
e,
Diego P.
Rocha
 a,
Edson
Nossol
a,
Edson
Nossol
 b,
Rodrigo A. A.
Munoz
b,
Rodrigo A. A.
Munoz
 *b,
Lucio
Angnes
*b,
Lucio
Angnes
 a and
Chandra Sekhar
Rout
a and
Chandra Sekhar
Rout
 *d
*d
aInstituto de Química, Universidade de São Paulo, Av. Prof. Lineu Prestes 748, 05508-000 São Paulo, SP, Brazil. E-mail: josuemartins@usp.br
bInstituto de Química, Universidade Federal de Uberlândia, Av. João Naves de Ávila 2121, Uberlândia, MG 2121, Brazil. E-mail: munoz@ufu.br
cDepartment of Physics, Berhampur University, Odisha, India
dCentre for Nano and Material Sciences, Jain University, Jain Global Campus, Jakkasandra, Ramanagaram, Bangalore-562112, India. E-mail: r.chandrasekhar@jainuniversity.ac.in; csrout@gmail.com
eInstituto de Química, Universidade Federal de Goiás, Av. Esperança s/n, 74690-900 Goiânia, GO, Brazil
First published on 9th December 2020
Abstract
The energy requirement of modern society increases every day. The depletion of the reserves of fossil fuel combined with the deleterious effects of CO2 in the atmosphere is forcing all the world to search for alternative ways of generation and storing energy. Many scientists around the world are pursuing different forms to produce and store energy. Solar and wind sources are a reality for production of electricity, but are not continuous and require storage devices. The development of batteries and hybrid supercapacitors of high energy and power density is of great importance to complement this requirement of energy storage. Rechargeable metal–air batteries which utilize oxygen electrocatalysis seem to be an ideal choice, once the source of energy is not intermittent as solar and wind energy and is based on oxygen bifunctional electrocatalysis of both oxygen reduction and O2 evolution reactions. In addition, water splitting allows the conversion and storage of solar/wind energy into chemical energy, generating fuels with high energy content. From this perspective, spinel MnCo2O4-based materials are promising structures for energy storage and conversion of energy. In this review, the use of low cost and abundant multifunctional materials for the development of supercapacitor devices and batteries was summarized. Completely, the design of electrocatalysts for water splitting and their capability to proportionate the tetra-electronic process of the oxygen reduction reaction are reviewed, including the main strategies in the preparation of these materials and considering their key multifunctional role in the way to a more sustainable society.
1. Introduction
With the increasing demand for environmentally friendly energy sources, alternatives have accelerated research on various renewable energy technologies such as fuel cells, metal–air batteries, and water-splitting devices as alternative energy production and storage systems.1However, there are several scientific and technological challenges which require great efforts in the search for a more sustainable society. Among the main challenges, it is easy to identify the scientific race for low-cost and abundant materials for the command of the tetra-protonic and tetra-electronic reaction mechanism of the oxygen evolution reaction (OER),2,3 a formidable challenge in the development of H2 fuel cells. In addition, electrochemical oxygen reduction (ORR) and OER reactions are two key processes that limit the efficiency of important energy conversion devices such as metal–air batteries (MABs) and electrolytic cells.4 On the other hand, the quest for much higher power and energy density devices, especially hybrid supercapacitors (HSCs), as alternatives to lithium-ion batteries (LIBs), has been the main objective of several research groups, as they can combine the outstanding power density of supercapacitive materials with the high energy density of battery-type materials into a single device.5
In this sense, researchers in materials science have strived to develop advanced and multifunctional materials for modern energy technologies, aiming to overcome the main challenges of energy conversion and storage. In fact, among the several recently studied materials, transition metal oxides (TMOs) have garnered attention due their high electronegativity, rich redox reactions and abundant density of active sites, low cost, environmental friendliness, and excellent electrochemical performance.6 For instance, recently some review articles reported the use of Co3O4 and Co3O4-containing electrode materials for supercapacitors7 and batteries.8 In one of these recent studies, Hu et al.9 summarized the proposed strategies for improving specific capacitance, cycling stability, multifunctional capabilities of Co3O4 based materials and development prospects of Co3O4-based supercapacitor materials, providing a certain direction for application of Co3O4 in supercapacitors in the future. Complementarily, Shi and co-workers8 discussed the synthesis and application of pure Co3O4 and its composites (Co3O4/C, Co3O4/graphene, Co3O4/metal oxide) in the field of LIBs. On the other hand, in the field of energy conversion, M. R. N. S. Hamdani, R. N. Singh, & P. Chartier (2010)10 reviewed the performance of Co3O4 and Co-based spinel oxides as electrocatalysts for the OER or ORR.
Similar to Co3O4 and Co3O4-based materials, manganese-containing TMOs have also been intensively reported for applications in energy technologies,6 especially those based on Mn3O4. For example, Zhu et al.11 reviewed the electrochemical properties and reaction principles of Mn3O4-based composites with carbon and other metal compounds for supercapacitor electrodes. In addition to the use in supercapacitors, Ubale and colleagues12 reported the main advances in the deposition, characterization, and applications of nanostructured manganese oxide thin films (NMOTFs) in LIBs, highlighting the structural and morphological studies. On the other hand, Tian and co-workers13 reported the emerging applications of a series of MnOx materials as highly efficient electrocatalysts for the OER, highlighting the reaction mechanisms, superiorities, and challenges of each type of MnOx for future applications in the highly exciting energy-conversion-related areas.
As already mentioned in the studies cited above, spinel materials with a typical chemical formula of AB2O4 have been widely recognized and considered in the energy storage field,14 and also as electrocatalysts in energy conversion devices. In fact, special attention has been given to spinel materials with bimetallic oxide structure, as they can result in materials with higher electrochemical activity, electrical conductivity, and more abundant redox reactions compared with monometallic oxides of A and B.15 For example, some reviews reported the recent progress in the use of the NiCo2O4 spinel in supercapacitors,16 batteries17 and sensors.18 More recently, Zhao et al.14 summarized the main advances of 2D spinel structured Co-based MCo2O4 (M = Co, Ni, Zn, Cu, Fe, and Mn) materials as integrated electrodes for supercapacitor (SC) applications, detailing other different nanomaterials and 2D spinel structured Co-based materials for this application.
To our knowledge, more than five hundred articles report the preparation and/or use of the MnCo2O4 spinel for various applications, especially for energy conversion and storage. Thus, this compound has been widely recognized as a promising, versatile, and cost-efficiently bifunctional non-noble-metal electrocatalyst, due to its high redox stability, the complementation and synergy of both transition metals (manganese and cobalt), and efficient variable valence states.19–22 As shown earlier, a few of the previous reviews have discussed the applications of Co3O4, Mn3O4 and NiCo2O4 spinels in energy storage, especially in supercapacitors and LIBs. However, as far as we know, there is no review work describing the promising results of MnCo2O4 in energy technologies. Therefore, in this review article we focus on the recent advances in MnCo2O4-based materials for energy applications and the main strategies used for the design of these materials (Scheme 1), including HSCs, LIBs and MABs, as well as the advancements achieved as electrocatalysts for water-splitting, more specifically for the hydrogen evolution reaction (HER) and OER. The pros and cons of using this spinel in the different devices are critically discussed. Finally, the evolving application of MnCo2O4 materials in the ORR is discussed, as well as the perspectives and future directions anticipated.
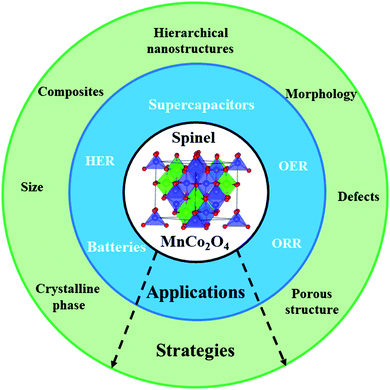 | ||
| Scheme 1 Illustration of the strategies and applications of MnCo2O4 spinels. The atomic structure of the MnCo2O4 inverse spinel structure in the center of the scheme was reproduced with permission from ref. 23. | ||
2. MnCo2O4 spinel: a supercapacitive or battery-type material?
The growing and fast demand for clean and sustainable energy storage devices has generated a scientific race for abundant and low-cost materials that can be used in high energy density devices, especially in high power density applications. However, this scientific race has also resulted in a great deal of confusion in the classification of supercapacitive and battery-like materials, especially in the distinction of “pseudocapacitive” and “battery” materials.24 In this sense, several review articles have recently been published in order to alert the scientific community on these misunderstandings.To clarify the confusion, Chodankar et al.24 reported a review article that serves as a guide, providing the meanings and correct performance metrics of different electrode materials and using the electrochemical signatures and quantitative kinetics analysis as a method to distinguish battery-type and pseudocapacitive materials. For instance, electrical double-layer capacitors (EDLCs) that store energy purely in the double-layer on a high surface area conductor25 show a typical electrochemical signature of a supercapacitive material (Fig. 1a), that is, a rectangular cyclic voltammogram (CV, Fig. 1b) and galvanostatic charge/discharge (GCD) profile in the form of a symmetrical triangle (Fig. 1c). Similarly, pseudocapacitive materials have quasi-rectangular CVs and quasi-triangular GCD curves, however the charge storage mechanisms involve either (a) redox reactions at or near the surface (intrinsic pseudocapacitors); or (b) intercalation-type reactions.24
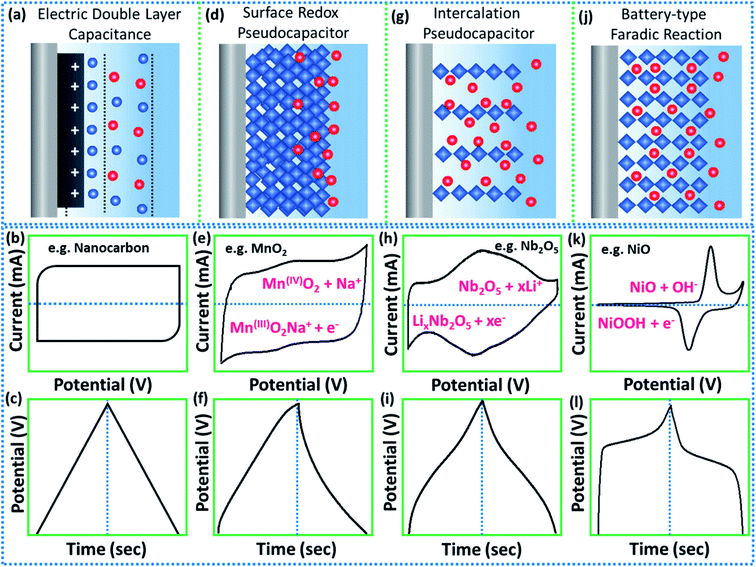 | ||
| Fig. 1 The schematic illustration of the energy storage mechanisms with their corresponding electrochemical signatures (representative shapes of CV and CD curves): (a–c) electrical double layer capacitance, (d–f) surface redox capacitance, (g–i) intercalation capacitance, and (j–l) faradaic battery-type. Reproduced with permission from ref. 24. Copyright © 2020 Wiley-VCH GmbH. | ||
Surface-redox pseudocapacitors, for example, are well represented by ruthenium (RuO2) and manganese oxides (MnO2). In fact, due to their fast proton and electron-conducting properties at the surface of the electrode (Fig. 1d), their electrochemical signatures resemble those of EDLCs, as shown in Fig. 1e and f. In contrast, some layered oxides, such as Nb2O5 and MoO3, can store energy by faradaic processes through the intercalation of electrolyte ions into the layers (Fig. 1g), especially in a nonaqueous electrolyte system, but without crystallographic phase changes. These materials are of the type “intercalation pseudocapacitors”, but they should not be confused and called redox pseudocapacitors. According to Chodankar et al.,24 a way to avoid this confusion is to carefully analyze the electrochemical features of intercalation pseudocapacitive materials and it was found that (i) they do not undergo phase transformations during intercalation, (ii) their peak potentials do not shift considerably with sweep rate, (iii) their current is linearly proportional to the sweep rate and (iv) their capacity does not vary significantly with charging time.
On the other hand, battery-type electrode materials have an electrochemical signature quite different from supercapacitive materials, since CVs display a couple of redox peaks (Fig. 1k) and plateau GCD profiles (Fig. 1l). This is due to the solid-state diffusion-controlled faradaic reactions characteristic of materials that present phase change of the electrode materials during the electrochemical process (Fig. 1j), such as oxides/hydroxides of Ni, Co, Cu, and Cd that react with hydroxide ions in alkaline media to store a charge.24
Then, is the MnCo2O4 spinel a supercapacitive or battery-type material? In the literature it is possible to find some studies that classify MnCo2O4 as a supercapacitive material, while others as battery-type. For example, V. Sannasi & K. Subbian26 reported the preparation of high-pseudocapacitance MnCo2O4 nanostructures, while S. G. Krishnan, M. H. A. Rahim & R. Jose27 reported the synthesis and characterization of MnCo2O4 cuboidal microcrystals as intercalation pseudocapacitors, however, both studies presented the characteristic electrochemical signature of a battery-like material, such as CVs with a couple of redox peaks and plateau GCD profiles. In addition, peak potentials shifted considerably with sweep rate and capacity varied significantly with charging time. Thus, although many studies classify MnCo2O4 as a supercapacitive material, in this review work it was considered as a battery-type material, and in many cases the energy stored in the form of specific charge (C g−1) was recalculated, since the average capacitance (F) was not constant throughout the potential window in the CVs.
In addition, it is also important to clarify that when assembling a battery-type electrode (ex.: MnCo2O4) with a supercapacitive-type electrode, a HSC is obtained, matching the advantages from both batteries and supercapacitors, and rendering them promising advanced energy storage devices for commercial applications.28 In fact, experimental and theoretical studies29 (shown below) demonstrated that MnCo2O4 has a superior electrical conductivity when compared to Co3O4, also showing greater storage capacity compared to other cobaltite spinels (MCo2O4; M = Ni,28 Cu,28 Zn30 and Co28,30) with good cycling lifespan.29 These characteristics demonstrate the promising possibilities of using these materials in high performance HSCs, as discussed below.
3. MnCo2O4-based materials for energy storage applications
3.1. Supercapacitors
In addition, it is important for a supercapacitor to have suitable fitting pore size distribution and large specific surface area, aiming to decrease the consumption of electrolyte by regulating the porous structure and morphology of the electrode, which determine the ion diffusion and conductivity, thereby affecting the capacitance of the supercapacitor. MnCo2O4 materials with different morphologies, such as spheres,36 granules,37 cuboidal microcrystals,27 nanoneedles,38 nanorods,39,40 cubes,26,41 nanosheets,42–44 nanocages,45 tunable porous structures,46 hollow spheres,47 and network-like porous structures,48,49 can be prepared and tested for their usefulness as supercapacitor electrodes. For example, 1D MnCo2O4 nanowire arrays showed a specific capacitance of 349.8 F g−1 at 1 A g−1 and an energy density of 35.4 W h kg−1 at a power density of 225 W kg−1.50 Similarly, specific capacitances of 1342 F g−1 at 1 A g−1 and 988 F g−1 at 20 A g−1 were observed for MnCo2O4 nanowires synthesized by Xu et al.51
Liu et al.29 reported a MnCo2O4 mesoporous nanowire array grown on nickel foam (NF) with a high specific capacitance. From Fig. 2a it is possible to observe that the nanowire has a mesoporous characteristic being formed by MnCo2O4 nanoparticles with a size distribution of ∼20 nm (Fig. 2b) and a surface area of 98.5 m2 g−1 (determined from N2 isotherms). In order to figure out the effect of Mn on the MnCo2O4 spinel the projected density of states and electronic band structures were determined and the results are shown in Fig. 2c and d, respectively. Those studies demonstrated that MnCo2O4 has a superior electrical conductivity when compared to Co3O4. In fact, MnCo2O4 presented a valence bond very near the Fermi level and a low bandgap of 0.35 eV at the G point, in contrast the Co3O4 presented a bandgap of 1.72 eV at the same point. Also, the electrochemical behavior of MnCo2O4 nanowires was superior to that of Co3O4. A specific capacitance of 2146 F g−1 at a current density of 1 A g−1 was observed for MnCo2O4 nanowires, while for Co3O4 the specific capacitance was 948 F g−1,29Fig. 2e. In addition, the MnCo2O4 nanowires presented an excellent capacitance retention of 92.1% even after 5000 cycles of charge–discharge process, as can be seen in Fig. 2f.
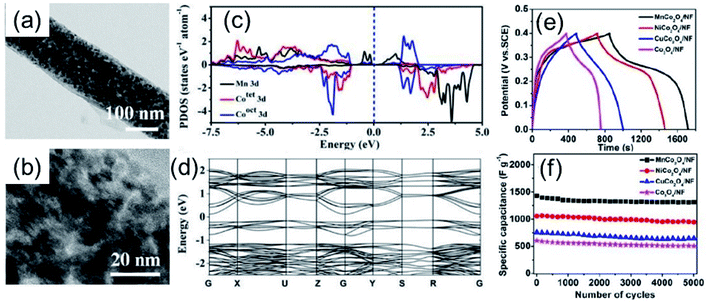 | ||
| Fig. 2 TEM (a) and HRTEM (b) images of MnCo2O4 nanowires, (c and d) projected density of states and electronic band structures of MnCo2O4. (e) GCD curves of MnCo2O4 nanowires (black, at 1 A g−1) and (f) cycling performance of MnCo2O4 nanowires (black, at 1 A g−1). Reproduced with permission from ref. 29. Copyright © Marketplace™, Royal Society of Chemistry. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Besides, the hybrid supercapacitor presented an energy density of 68.2 W h kg−1 at 749.2 W kg−1 power density.
000 cycles. Besides, the hybrid supercapacitor presented an energy density of 68.2 W h kg−1 at 749.2 W kg−1 power density.
Liu et al.59 reported the synthesis of a hierarchical MnCo2O4 nanowire@MnO2 sheet core shell nanostructure showing an energy density of 85.7 W h kg−1 at a power density of 800 W kg−1. Zheng and coauthors60 also reported a hierarchical MnCo2O4@MnO2 core–shell nanowire array exhibiting an energy density of 135.6 W h kg−1 at a power density of 513 W kg−1.
Smarter integrated designs combined with different oxide materials are also reported, such as MnO2,61 CoO,62 NiWO4,63 ZnO,64 NiO,65 CoCo2O4,66 CuCo2O4,67 NiCo2O4,68 and CoMnO4.69 These types of structures possess many competitive advantages, including improvement of electrical conductivity, high electron aggregation efficiency, rich approachable electroactive sites, and even excellent synergetic effects or multifunctional properties of the nanostructure components.70
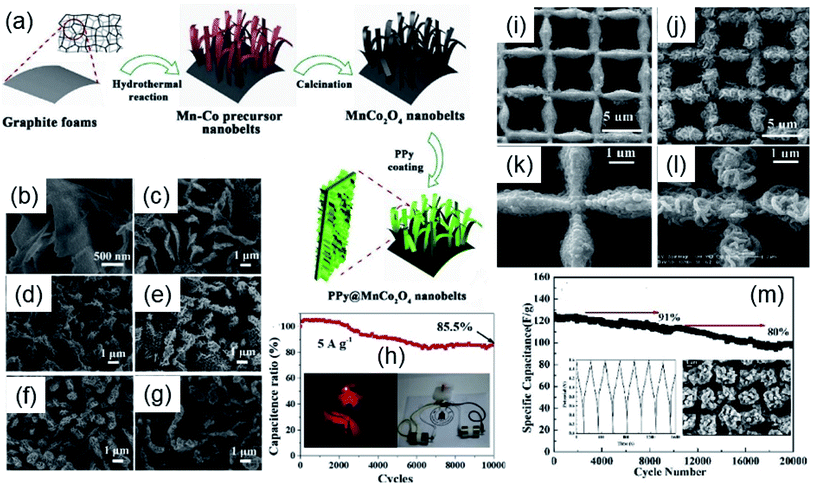 | ||
| Fig. 3 Scheme of the synthesis process of PPy@MnCo2O4/GNF (a), SEM images of the PPy@MnCo2O4/GNF-n (n = 1–6) (b–g) and cycling stability performance of the hybrid supercapacitor PPy@MnCo2O4/GNF-5//a-MEGO (h). Reproduced with permission from ref. 72. Copyright © 2018 Elsevier B.V. All rights reserved. SEM images of nanographene@MECN at low (i) and high magnification (k) and B-n-MnCo2O4@MECN at low (j) and high magnification (l). Stability electrochemical performance of the hybrid supercapacitor B-n-MnCo2O4@MECN//AC@Ni foam (m). Reproduced with permission from ref. 77. Copyright © 2020 Elsevier B.V. All rights reserved. | ||
Furthermore, a HSC was built with MnCo2O4@PPy/GNF-5 and activated microwave exfoliated graphite oxide (a-MEGO) as positive and negative electrodes, respectively. The HSC showed an energy density of 25.7 W h kg−1 and a power density of 16.1 kW kg−1, besides a capacitance retention of 85.5% after incredible 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 3h). Hu et al.73 also reported a composite based on PPy decorated by MnCo2O4 urchins showing an energy density of 0.785 mW h cm−1 at a power density of 7.49 W cm−1 as the positive electrode for supercapacitors.
000 cycles (Fig. 3h). Hu et al.73 also reported a composite based on PPy decorated by MnCo2O4 urchins showing an energy density of 0.785 mW h cm−1 at a power density of 7.49 W cm−1 as the positive electrode for supercapacitors.
Wu and coworkers77 developed a new bifunctional composite based on MnCo2O4/nanographene (B-n-MnCo2O4) prepared on a macroporous electrically conductive network (MECN) as an electrode material for supercapacitors and sodium ion batteries. The 3D structure of nanographene coating MECN and B-n-MnCo2O4@MECN can be seen in Fig. 3i–l. Fig. 3i and k show nanographene layers with a lateral size of 50–200 nm and when the B-n-MnCo2O4 was incorporated onto the MECN (Fig. 3j and l) the 3D nanostructure displayed a uniform diameter of ∼50 nm. This 3D interconnected morphology seems to be very interesting since it can improve the electronic conductivity, in addition to facilitating electrochemical reactions, because of its large surface area. The B-n-MnCo2O4@MECN presented a high specific capacitance of 7.02 F cm−2 (2341 F g−1) at 3 mA cm−2. The specific capacitance of B-n-MnCo2O4@MECN is much larger than that of Mn3O4/nanographene@MECN (2.7 F cm−2) and Co3O4/nanographene@MECN (2.2 F cm−2) at the same current density, which highlights the presence of MnCo2O4 in the composite.
Also, a HSC was built with B-n-MnCo2O4@MECN and AC@Ni foam as positive and negative electrodes, respectively. The device provided an outstanding long lifetime and stability as well. Even after incredible 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles the device showed a capacitance retention of 80%, as can be seen in Fig. 3m. The long lifetime can be attributed to good adhesion between all the components and good electrical contact between B-n-MnCo2O4 and MECN as the current collector.
000 charge–discharge cycles the device showed a capacitance retention of 80%, as can be seen in Fig. 3m. The long lifetime can be attributed to good adhesion between all the components and good electrical contact between B-n-MnCo2O4 and MECN as the current collector.
Most of the materials previously-mentioned were deposited on Ni foam as the conductive substrate,27,57,60,65 which did not require the addition of polymer binders, and promoted rapid electron transport between the active material and current collector, thereby resulting in a substantially efficient substrate, due to the high electrical conductivity (1.43 × 107 Ω−1) and thermal conductivity (90.7 W mK−1) of Ni.90 Nevertheless, other conductive substrates and current collectors have also been employed, such as carbon aerogels,81 graphite foam,72 indium-doped tin oxide,44 activated carbon,78 microporous electrically conductive networks,77 graphite paper,59,67,95 carbon fiber paper96 and copper foil.35Table 1 presents the performance of different supercapacitor materials coupled with different types of electrodes based on MnCo2O4.
| Type | Material | Specific capacitance (F g−1) | Specific capacity (C g−1) | Potential window (V vs.) | Retention (%), rate capability (A g−1) | Cycling stability | Highest energy density (W h kg−1) | Highest power density: (kW kg−1) | Negative electrode material | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| a AC = activated carbon; a-MEGO = activated microwave exfoliated graphite oxide; CFs = carbon fibres; rGO = reduced graphene oxide; NF = nickel foam; N-CNTs = N-doped carbon nanotubes; PPy = polypyrrole; rMnCo2O4@rMnO2 = reduced core–shell structured MnCo2O4@MnO2. * By cyclic voltammetry. | ||||||||||
| Pristine | Flake-like MnCo2O4 | 1487 F g−1 at 1 A g−1 | 594.8 C g−1 | 0.0–0.4 V (vs. SCE) | — | 93.3% (2000 cycles) | — | — | — | 35 |
| MnCo2O4 | 405 F g−1 at 1 mA cm−2 | — | 0–0.4 V (vs. SCE) | 67.9%, (5–40 mA cm−2) | 95.1% (1000 cycles) | — | — | — | 37 | |
| MnCo2O4 nanorods | 845 F g−1 at 1 A g−1 | 422.5 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 51.6%, (1–20 A g−1) | 90.2% (2000 cycles) | 53.7 | 8 | rGO/NF | 39 | |
| MnCo2O4 nanorods | 718.75 F g−1 at 0.5 A g−1 | 287.5 C g−1 | 0.0–0.4 V (vs. Ag/AgCl) | — | 100% (1000 cycles) | — | — | — | 40 | |
| MnCo2O4 hollow spheres | 0.8 F cm−2 at 2 mA cm−2 | 0.32 C cm−2 | 0.0–0.4 V (vs. SCE) | — | 99% (2000 cycles) | 0.052 mW h cm−3 | 320 mW cm−3 | AC/CFs | 47 | |
| Porous sphere MnCO2O4 | 1044 F g−1 at 0.5 A g−1 | 574.2 C g−1 | 0.0–0.55 V (vs. Hg/HgO) | 68.9%, (1–20 A g−1) | 133.3% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
42.27 | 0.4 | Starch-derived carbon foam | 36 | |
| Network-like porous MnCo2O4 | 647.42 F g−1 at 1 A g−1 | 323.71 C g−1 | 0.1–0.6 V (vs. Hg/HgO) | 70.67%, (1–10 A g−1) | 93.68% (3000 cycles) | — | — | — | 48 | |
| MnCO2O4 nanowires | 2146 F g−1 at 1 A g−1 | 858.4 C g−1 | 0.0–0.4 V (vs. SCE) | — | 92.1% (5000 cycles) | 29.3 | 8 | AC | 29 | |
| MnCO2O4 nanowires | 405 F g−1 at 1 A g−1 | 202.5 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 51.4%, (1–10 A g−1) | 91% (4000 cycles) | — | — | — | 97 | |
| MnCO2O4 nanowires | 1342 F g−1 at 1 A g−1 | 738.1 C g−1 | 0.0–0.55 V (vs. Hg/HgO) | 73.6%, (1–20 A g−1) | — | — | — | — | 51 | |
| MnCO2O4 nanowires | 349.8 F g−1 at 1 mV s−1 | 157.4 C g−1 | 0.0–0.45 V (vs. Ag/AgCl) | 94%, (1–20 A g−1) | 94% (4000 cycles) | 33.3 | 4.5 | — | 50 | |
| MnCo2O4 nanoparticles | 1068.5 F g−1 at 1 A g−1 | 427.2 C g−1 | 0.0–0.4 V (vs. SCE) | 50%, (1–8 A g−1) | 90% (2000 cycles) | — | — | — | 26 | |
| Chestnut-like MnCo2O4 nanoneedles | 1535 F g−1 at 1 A g−1 | 921 C g−1 | 0.0–0.6 V (vs. SCE) | 61.8%, (1–10 A g−1) | 94.3% (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
∼60.4 | ∼0.375 | Graphene/NF | 38 | |
| MnCo2O4 nanosheets | 2000 F g−1 at 0.5 A g−1 | 1000 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 57.5%, (0.5–20 A g−1) | 92.3% (5000 cycles) | 73.95 | 15 | rGO | 42 | |
| Nanocage MnCo2O4 | 1763 F g−1 at 1 A g−1 | 969.65 C g−1 | 0.0–0.55 V (vs. Hg/HgO) | — | 95% (4500 cycles) | 54.15 | 0.324 | Nanocage MnCo2O4 | 45 | |
| Flower-like MnCo2O4 | 249.3 F g−1 at 0.5 A g−1 | 174.5 C g−1 | −0.3–0.4 V (vs. Hg/HgO) | 78.8%, (0.5–5 A g−1) | 93.6% (2000 cycles) | — | — | — | 98 | |
| Flower-like MnCo2O4 | 571 F g−1 at 0.5 A g−1 | 285.5 C g−1 | −0.1–0.4 V (vs. Hg/HgO) | 87.7%, (0.5–5 A g−1) | 96.1% (2000 cycles) | — | — | — | 99 | |
| MnCo2O4 cubes | 480.5 F g−1 at 1 A g−1 | 264.3 C g−1 | 0.0–0.55 V (vs. Hg/HgO) | 75.7%, (1–25 A g−1) | 96.6% (3000 cycles) | — | — | — | 41 | |
| MnCo2O4 cuboidal microcrystals | 600 F g−1 at 0.5 A g−1 | 300 C g−1 | 0.0–0.5 V (vs. Ag/AgCl) | 55.7%, (0.5–5 A g−1) | 132% (3000 cycles) | — | — | — | 27 | |
| MnCo2O4 | 270 F g−1 at 10 mV s−1 | — | −1.2–1.5 V (vs. Ag/AgCl) | — | 92.4% (1000 cycles) | 14.85 | 0.495 | — | 49 | |
| Porous MnCo2O4 | 151 F g−1 at 5 mV s−1 | — | 0–0.5 V (vs. SCE) | 83.6%, (0.1–5 A g−1) | — | — | — | — | 95 | |
| MnCo2O4 nanosheets | — | 282 C g−1 at 1 A g−1 | 0–0.55 V (vs. SCE) | 25.2%, (1–10 A g−1) | 84% (1500 cycles) | 2.55 | 37.57 | FeMn2O4 | 43 | |
| MnCo2O4 nanorods | 187.0 F g−1 at 0.25 A g−1 | 51.9 C g−1 | 0.0–0.4 V (vs. Ag/AgCl) | 8%, (0.25–30 A g−1) | 90% (2000 cycles) | 12.77 | 2.520 | AC | 100 | |
| MnCo2O4 nanosheets | 250 F g−1 at 0.25 A g−1 | 100 C g−1 | 0.0–0.4 V (vs. Ag/AgCl) | 10.8%, (0.25–10 A g−1) | 95% (1000 cycles) | 10.04 | 5.2 | — | 44 | |
| MnCo2O4-metal oxide | Co3O4@MnCo2O4 | 736.5 F g−1 at 1 mA cm−2 | — | 0–0.5 V (vs. SCE) | — | 79.95% (3000 cycles) | — | — | — | 53 |
| MnCo2O4@Co3O4 | — | 1440 C cm−2 at 1 mA cm−2 | 0.0–0.45 V (vs. SCE) | — | 101.23% (8000 cycles) | 31 | 0.208 | AC | 54 | |
| MnCo2O4@CoMnO4 | 2115.38 F g−1 at 1.1 A g−1 | 1100 C g−1 | 0.0–0.52 V (vs. Hg/HgO) | 47.4%, (1.1–6.6 A g−1) | 119% (5000 cycles) | 37.5 | 0.527 | AC | 69 | |
| MnCo2O4@CoCo2O4 | 614 F g−1 at 1 A g−1 | 276.3 C g−1 | 0.0–0.45 V (vs. SCE) | 76.9%, (1–10 A g−1) | 77.5% (5000 cycles) | — | — | — | 66 | |
| MnCo2O4@NiMoO4 | 1718 F g−1 at 1 A g−1 | 859 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 69.8%, (1–8 A g−1) | 84% (6000 cycles) | 42.3 | 0.797 | AC | 56 | |
| CuCo2O4/MnCo2O4 | 1434 F g−1 at 0.5 A g−1 | 860.4 C g−1 | 0.0–0.6 V (vs. SCE) | 56.6%, (0.5–15 A g−1) | 81.4% (5000 cycles) | 42.1 | 0.4 | Graphene/NF | 67 | |
| MnCo2O4@NiMoO4 | 1244 F g−1 at 1 A g−1 | 746.4 C g−1 | 0.0–0.6 V (vs. SCE) | 91%, (1–10 A g−1) | 81% (2500 cycles) | 42 | 0.8 | AC | 57 | |
| NiCo2O4–MnCo2O4 | 1152 F g−1 at 1 A g−1 | 576 C g−1 | 0.0–0.5 V (vs. Ag/AgCl) | 72.2%, (1–10 A g−1) | 95.4% (3000 cycles) | 40 | 0.2 | — | 68 | |
| MnO2/MnCO2O4 | 497 F g−1 at 0.5 A g−1 | 248.5 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 62.8%, (0.5–10 A g−1) | 60% (5000 cycles) | — | — | — | 61 | |
| MnCo2O4@NiO | 508.3 F g−1 at 2 A g−1 | 279.6 C g−1 | 0.0–0.55 V | 67.7%, (0.5–6 A g−1) | 89.7% (2000 cycles) | — | — | — | 65 | |
| MnCo2O4@NiWO4 | 5.09 F cm−2 at 1 mA cm−2 | 2.54 C cm−2 | 0.0–0.5 V (vs. Hg/HgO) | 26.1%, (1–20 mA cm−2) | 96% (5000 cycles) | 0.23 mW h cm−2 | 2.66 mW cm−2 | AC | 63 | |
| CoO/MnO2/MnCO2O4 composite nanowires | 1650 F g−1 at 1 A g−1 | 660 C g−1 | 0.0–0.4 V (vs. Ag/AgCl) | 60.0%, (1–15 A g−1) | — | 90 | 10 | CoO/MnO2/MnCO2O4 composite nanowires | 62 | |
| rMnCo2O4@rMnO2 | 3.39 F cm−2 at 3 mA cm−2 | 0.4 mA h cm−2 | 0.0–0.4 V (vs. SCE) | 48.9%, (3–60 mA cm−2) | 92.5% (3000 cycles) | 32.4 | 4.524 | AC | 101 | |
| MnCo2O4@MnO2 core–shell arrays | 2262 F g−1 at 1 A g−1 | 904.8 C g−1 | 0.0–0.4 V (vs. SCE) | 48.8%, (1–20 A g−1) | 87.1% (5000 cycles) | 34.7 | 24 | Graphene/NF | 59 | |
| ZnO@MnCo2O4 | 631.2 F g−1 at 1 A g−1 | 504.9 C g−1 | −0.2–0.6 V (vs. SCE) | 31.7%, (1–10 A g−1) | 92.3% (1000 cycles) | 56.10 | 0.4 | — | 64 | |
| NiCo2O4–MnCo2O4 rose-like composite | 1100 F g−1 at 1 A g−1 | 440 C g−1 | 0.0–0.4 V (vs. SCE) | 69.1%, (1–10 A g−1) | — | — | — | — | 102 | |
| MnCo2O4@MnMoO4 core–shell | — | 885 C g−1 at 3 A g−1 | 0.0–0.4 V (vs. SCE) | 71%, (3–30 A g−1) | 95% (5000 cycles) | 49.4 | 0.815 | AC | 55 | |
| MnCo2O4-conducting polymer | MnCo2O4@PPy/GNF | 2933 F g−1 at 2 A g−1 | 1466.5 C g−1 | 0.0–0.5 V (vs. Ag/AgCl) | 55.3%, (2–20 A g−1) | 86.7% (5000 cycles) | 78.2 | 1.121 | AC | 32 |
| MnCo2O4@PPy | — | 2.862 mA h cm−2 at 1 mA cm−2 | 0.0–0.5 V (vs. Hg/HgO) | 84.5%, (1–10 mA cm−2) | 88% (2000 cycles) | 0.785 mW h cm−3 | 7.49 mW cm−3 | AC | 73 | |
| Polypyrrole@MnCo2O4/graphite foam | 2364 F g−1 at 1 A g−1 | 1182 C g−1 | −0.1–0.4 V (vs. Ag/AgCl) | 55.2%, (1 to 50 A g−1) | 85.3% (1000 cycles) | 25.7 | 16.1 | a-MEGO | 72 | |
| MnCo2O4-carbon based composites | MnCo2O4/graphene | 503 F g−1 at 1 A g−1 | 201.2 C g−1 | 0.0–0.4 V (vs. SCE) | 65.5%, (1–20 A g−1) | 97.4% (5000 cycles) | — | — | — | 76 |
| Flower-like MnCo2O4/rGO | 923.97 F g−1 at 1 A g−1 | 739.2 C g−1 | 0.0–0.8 V (vs. Ag/AgCl) | — | 111% (5000 cycles) | 82.13 | 0.399 | Carbon black | 85 | |
| MnCo2O4@nitrogen doped graphene@ MnO2 | 1170 F g−1 at 1 A g−1 | 585 C g−1 | 0.0–0.5 V (vs. Ag/AgCl) | 69.9%, (1–20 A g−1) | 87.6% (5000 cycles) | 48.5 | ∼0.808 | Nitrogen doped graphene hydrogel | 33 | |
| C@MnCo2O4 | 728.4 F g−1 at 1 A g−1 | 364.2 C g−1 | 0.0–0.5 V (vs. Ag/AgCl) | 71.3%, (1–10 A g−1) | — | 25.5 | 0.856 | — | 79 | |
| MnCo2O4@activated carbon | — | 443.5 C g−1 at 0.5 A g−1 | −1.0–0.4 V (vs. SCE) | — | 66.95% (5000 cycles) | — | — | — | 78 | |
| MnCo2O4 graphene quantum dots | 1625 F g−1 at 1 A g−1 | 812.5 C g−1 | 0.0–0.5 V (vs. Hg/HgO) | 80%, (0.5–10 A g−1) | 80% (5000 cycles) | 46 | 0.066 | rGO | 84 | |
| B-n-MnCo2O4@MECN | 7.02 F cm−2 at 3 mA cm−2 | — | −0.1–0.45 V (vs. Hg/HgO) | 31%, (3–20 mA cm−2) | 98% (5000 cycles) | 50.5 | 6.4 | AC/NF | 77 | |
| Other MnCo2O4-based composite materials | MnCo2O4@Co(OH)2 | 1185 C g−1 at 1 A g−1 | 0–0.5 V (vs. SCE) | 78%, (1–20 A g−1) | 96% (5000 cycles) | 67.2 | 0.8 | N-CNTs@rGO | 91 | |
| MnCo2O4@Ni(OH)2 | 2154 F g−1 at 5 A g−1 | 969.3 C g−1 | 0.0–0.45 V (vs. SCE) | 32.6%, (5–20 A g−1) | 90% (2500 cycles) | 48 | 14.9 | MnCo2O4@Ni(OH)2 | 92 | |
| MnCo2O4@Ni3S2 core–shell heterostructures | 2807 F g−1 at 3 A g−1 | 1122.8 C g−1 | 0.0–0.4 V (vs. SCE) | 69.0%, (3–30 A g−1) | 92% (5000 cycles) | — | — | — | 93 | |
| MnCo2O4@NiMn | 3063 F g−1 at 3 A g−1 | 1378.3 C g−1 | 0.0–0.45 V (vs. SCE) | 75.6%, (3–30 A g−1) | 94.7% (5000 cycles) | 51.9 | 0.8 | AC | 94 | |
| Dual-MnCo2O4/Ni | 2265 F g−1 at 1.7 A g−1 | 283 mA h g−1 | 0–0.45 V (vs. Ag/AgCl) | 70%, (2–10 mA cm−2) | 85% (2000 cycles) | — | — | — | 88 | |
| MnCo2O4@CoS | 1607.4 F g−1 at 0.8 A g−1 | 723.3 C g−1 | 0.0–0.45 V (vs. SCE) | — | 91.5% (5000 cycles) | 55.1 | 0.477 | AC | 86 | |
3.2. Batteries
In order to improve the electrochemical performance of MnCo2O4 structures as anodic materials in LIBs, morphological modifications and synthesis methods have been the focus of investigations.107–113 One of these strategies has been focused on the preparation of porous MnCo2O4 materials114,115 and on the development of diverse substrates as current collectors (carbon cloth,116–118 Ni foam113 and copper foil115,119) with robust adhesion to obtain a binder-free anode material. Li et al.120 first developed a two-step method to prepare uniform hollow MnCo2O4 submicrospheres with multilevel interiors (mesoporous, hollow, yolk–shell, shell-in-shell, and yolk-in-double-shell spheres). The yolk–shell morphology (Fig. 4a) showed the best performance among these multilevel interior structures, with an initial discharge capacity of 1425 mA h g−1 at a current density of 400 mA g−1 (Fig. 4b). Huang et al.121 also fabricated spherical yolk–shell MnCo2O4 powders (Fig. 4c) by a hydrothermal method followed by a thermal treatment, with an initial discharge capacity of 1445 mA h g−1 at 0.2 A g−1 and capacity retention of ∼860.0 mA h g−1 after 40 cycles at 0.2 A g−1 (Fig. 4d). The literature reports the preparation of different MnCo2O4 porous based structures for anode electrodes, such as spheres,122,123 yolk–shell microspheres124 (Fig. 4e and f), microflowers,125 hydrangea-like structures,126 and dumbbell-shaped structures.127
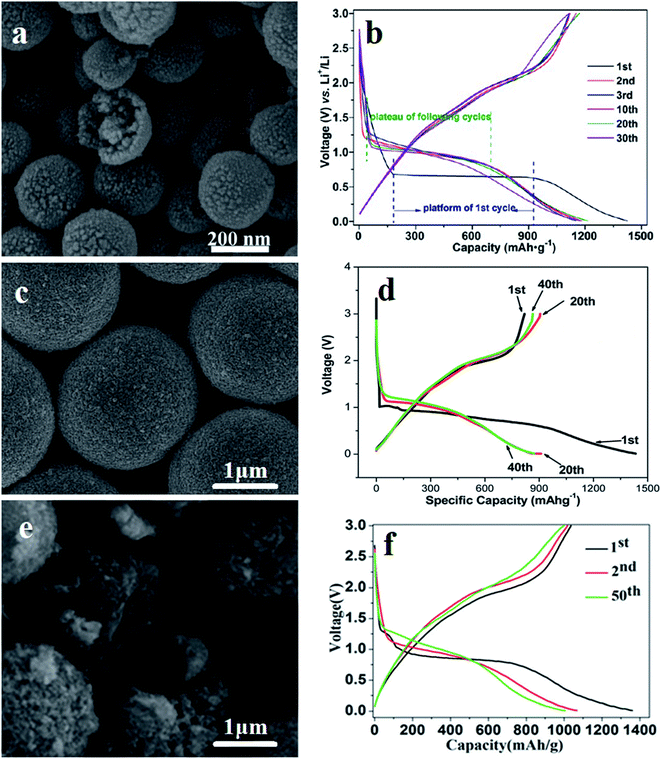 | ||
| Fig. 4 Scanning electron microscopy images (SEM) and galvanostatic charge–discharge profiles (GCD) of yolk–shell MnCo2O4. SEM images of (a) hollow MnCo2O4 submicrospheres. (c) Hierarchical porous MnCo2O4 yolk–shell microspheres and (e) spherical yolk–shell MnCo2O4 powders. GCD curves of (b) hollow MnCo2O4 submicrospheres, (d) hierarchical porous MnCo2O4 yolk–shell microspheres and (f) spherical yolk–shell MnCo2O4 powders. (a) and (b) adapted with permission from ref. 120. Copyright © 2014, American Chemical Society. (c and d) adapted with permission from ref. 121. Copyright © Marketplace™, Royal Society of Chemistry. (e and f) adapted with permission from ref. 124. Copyright © Marketplace™, Royal Society of Chemistry. | ||
Fu et al.106 reported the preparation of microspheres of MnCo2O4 by a calcination-free method; in this work, two kinds of MnCo2O4 crystals with different exposed facets of (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (1
0) and (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) were synthesized, presenting two different morphologies, particle-assembled and sheet-assembled microspheres, respectively. The anode was evaluated, and the microspheres delivered a capacity of 722 mA h g−1 after 25 cycles at a current density of 200 mA g−1, and capacities up to 553 and 320 mA h g−1 after 200 cycles at a current density of 400 and 900 mA g−1, respectively.
) were synthesized, presenting two different morphologies, particle-assembled and sheet-assembled microspheres, respectively. The anode was evaluated, and the microspheres delivered a capacity of 722 mA h g−1 after 25 cycles at a current density of 200 mA g−1, and capacities up to 553 and 320 mA h g−1 after 200 cycles at a current density of 400 and 900 mA g−1, respectively.
Huang and colleagues128 proposed a novel core–shell ellipsoidal MnCo2O4 powder with a desired micro-/nano-structure and unique concentration gradient. The battery tests demonstrated excellent values of initial discharge capacities (1433.3 mA h g−1 at 0.1 A g−1 and 1248.4 mA h g−1 at 0.4 A g−1), capacity retention (∼900.0 mA h g−1 after 60 cycles at 0.1 A g−1) and rate performance (∼620.0 mA h g−1 after 50 cycles at 0.4 A g−1).
Developing composites of MnCo2O4 with other materials is an important strategy to improve the performance of LIBs.129,130 A 3D sandwich-shape graphene based nanocomposite intercalated with double-shelled hollow MnCo2O4 spheres as an anode material for LIBs has been synthesized, showing a rate capability of 538 mA h g−1 at a current density of 1000 mA g−1 and outstanding cycle performance, with a capability of 703 mA h g−1 after 100 cycles at 200 mA g−1.131 The literature also reports the synthesis of MnCo2O4 containing nickel,132,133 Co3O4,119 CoO,118 MnO2,134 TiO2,135 and NiCo2O4.102 Huang and coauthors136 designed a MnCo2O4@N-doped carbon@MnO2 three layered core shell octahedron as an anode material for Li-ion storage, which displayed a discharge capacity of 894 mA h g−1 at a current density of 500 mA g−1 after 120 cycles. Even at a high current density of 1000 mA g−1, the discharge capacity remained at 839 mA h g−1 after 600 cycles.
Some conductive substrates have also served as current collectors to further improve the electrochemical performance of electrode materials, including carbon materials and conductive polymers, which have been mixed with MnCo2O4 structures,137,138 such as graphene,139 CNTs,140 carbon cloth116,117 and PPy.141 Hence, the construction of composites with the combination of two or more different materials has been proved as a promising strategy to boost the electrochemical performance of MnCo2O4. Due to the electronic conductivity and the specific surface area, rGO has been considered for the formation of composites with MnCo2O4. Fan et al.142 reported the synthesis of MnCo2O4/rGO composites with an initial discharge capacity of 1657 mA h g−1 at a current density of 0.1 A g−1, and a reversible capacity of 791 mA h g−1 at 0.2 A g−1 for 100 cycles. A MnCo2O4@PANi-rGO composite was also synthesized by Huang et al.,143 with a discharge capacity of 745 mA h g−1 and a coulombic efficiency of 100% after 1050 cycles at a current density of 500 mA g−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Flower-like MnCo2O4 synthesized by a co-precipitation method exhibited a discharge capacity of 244 mA h g−1 after 40 cycles at 50 mA h g−1, which corresponds to 77.1% compared with the second discharge capacity cycle.145Table 2 summarizes the performance of different cells of metal ion batteries coupled with different types of electrodes based on MnCo2O4.
000 cycles. Flower-like MnCo2O4 synthesized by a co-precipitation method exhibited a discharge capacity of 244 mA h g−1 after 40 cycles at 50 mA h g−1, which corresponds to 77.1% compared with the second discharge capacity cycle.145Table 2 summarizes the performance of different cells of metal ion batteries coupled with different types of electrodes based on MnCo2O4.
| Battery type | Material type | Material | Initial discharge (mA h g−1) | Potential window (V vs.) | Reversible capacity (Nth) mA h g−1 | Stability (cycle numbers) | Ref. |
|---|---|---|---|---|---|---|---|
| a rGO = reduced graphene oxide, MWCNT = multi wall carbon nanotube, PANi = polyaniline, and NC = N-doped carbon. | |||||||
| Lithium ion batteries (LIBs) | Pristine MnCo2O4 | MnCo2O4 yolk–shell microspheres | 1035.8 at 0.1C | 0.01–3 Li/Li+ | 691.3 (500) at 1C | — | 124 |
| Core–shell ellipsoidal MnCo2O4 | 1433.3 at 0.1 A g−1 | 0.01–3 Li/Li+ | 750 (70) at 100 mA g−1 | — | 128 | ||
| Micro-octahedral MnCo2O4 | 1438 at 300 mA g−1 | 0.01–3 Li/Li+ | 720.4 (200) at 300 mA g−1 | 88% (200 cycles) | 110 | ||
| MnCo2O4 nanoflakes | 1795 at 50 mA g−1 | 0.01–3 Li/Li+ | 925 (50) at 100 mA g−1 | — | 109 | ||
| MnCo2O4 microspheres | 1473 at 60 mA g−1 | 0.01–3 Li/Li+ | 533 (200) at 400 mA g−1 | — | 106 | ||
| MnCo2O4 cubic microcrystals | 1443 at 100 mA g−1 | 0.01–3 Li/Li+ | — | 28% (100 cycles) | 104 | ||
| MnCo2O4 needle-shaped | 1326 at 60 mA g−1 | 0.01–3 Li/Li+ | 368 (50) at 60 mA g−1 | 55% (50 cycles) | 107 | ||
| MnCo2O4 hollow spheres | 1561 at 200 mA g−1 | 0.01–3 Li/Li+ | 1023 (200) at 60 mA g−1 | — | 122 | ||
| MnCo2O4 hollow submicrospheres | 1119 at 400 mA g−1 | 0.01–3 Li/Li+ | 800 (100) at 100 mA g−1 | — | 120 | ||
| MnCo2O4 quasi-hollow microspheres | 1473 at 200 mA g−1 | 0.01–3 Li/Li+ | 610 (100) at 400 mA g−1 | — | 123 | ||
| MnCo2O4 | 900 at 60 mA g−1 | 0.005–3 Li/Li+ | 816 (50) 60 mA g−1 | — | 111 | ||
| MnCo2O4 microspheres | 1425.8 at 400 mA g−1 | 0.005–3 Li/Li+ | 1033.3 (200)/400 mA g−1 | 74.2% (200 cycles) | 105 | ||
| Dumbbell-shaped porous MnCo2O4 | 2073 at 200 mA g−1 | 0.01–3 Li/Li+ | 955(180) at 200 mA g−1 | 46% (180 cycles) | 127 | ||
| Erythrocyte like MnCo2O4 | 1538 at 200 mA g−1 | 0.01–3 Li/Li+ | 960 (100) at 200 mA g−1 | 77.6% (100 cycles) | 112 | ||
| Mesoporous MnCo2O4 microflowers | 1465.1 at 100 mA g−1 | 0.01–3 Li/Li+ | 732 (50) at 100 mA g−1 | — | 125 | ||
| MnCo2O4 nanospheres | 1184.8 at 400 mA g−1 | 0.01–3 Li/Li+ | 749.1 (50) at 200 mA g−1 | 89.8% (50 cycles) | 108 | ||
| Porous MnCo2O4 | 1750.0 at 400 mA g−1 | 0.01–3 Li/Li+ | 690.1 (100) at 0.5C | — | 114 | ||
| Flake-like MnCo2O4 | 1460 at 100 mA g−1 | 0.01–3 Li/Li+ | 952 (100) at 100 mA g−1 | 89% (100 cycles) | 35 | ||
| MnCo2O4 nanosheets | 3.9 mA h cm−2 at 800 μA cm−2 | 0.01–3 Li/Li+ | 3.0 mA h cm−2 (60) at 800 μA cm−2 | — | 116 | ||
| Porous MnCo2O4 nanosheets | 1044 at 0.2 A g−1 | 0.01–3 Li/Li+ | 848 (200) at 0.2 A g−1 | 81% (200 cycles) | 113 | ||
| Porous MnCo2O4 microspheres | 1034 at 1000 mA g−1 | 0.01–3 Li/Li+ | 740 (1000) at 1000 mA g−1 | — | 115 | ||
| Yolk–shell MnCo2O4 microspheres | 1445.1 at 0.2 A g−1 | 0.01–3 Li/Li+ | 860 (40) at 0.2 A g−1 | — | 121 | ||
| MnCo2O4 | 1220 at 0.1C | 0.1–3 Li/Li+ | 907 (50) at 0.5C | 90% (50 cycles) | 146 | ||
| MnCo2O4 nanotubes | 1211.9 at 0.5 A g−1 | 0.01–3 Li/Li+ | 701.4 (320) at 500 mA g−1 | — | 147 | ||
| Porous hydrangea-like MnCo2O4 | 1232 at 0.1 A g−1 | 0.01–3 Li/Li+ | 930 (100) at 0.1 A g−1 | 87% (100 cycles) | 126 | ||
| MnCo2O4–metal oxide composite | CoO/MnCo2O4.5 nanorods | 1183 at 200 mA g−1 | 0.01–3 Li/Li+ | 1030 (120) at 200 mA g−1 | 87% (120 cycles) | 118 | |
| MnCo2O4@NC@MnO2 | 1380 at 500 mA g−1 | 0.01–3 Li/Li+ | 894 (120) at 500 mA g−1 | — | 136 | ||
| NiO–MnCo2O4–Ni6MnO8 | 1284 at 30 mA g−1 | 0.01–3 Li/Li+ | 779 (120) at 1C | — | 129 | ||
| Co3O4–MnCo2O4 powder, 20–50 nm | 1781 at 50 mA g−1 | 0.01–3 Li/Li+ | 1250 (200) at 1000 mA g−1 | 68.2% (200 cycles) | 119 | ||
| MnCo2O4–TiO2 microspheres | 1396.9 at 100 mA g−1 | 0.01–3 Li/Li+ | 1271 (200) at 100 mA g−1 | 91% (200 cycles) | 135 | ||
| Porous MnCo2O4@MnO2 | 1927.8 at 100 mA g−1 | 0.0–3 Li/Li+ | 1162.8 (200) at 1 A g−1 | 96% (200 cycles) | 134 | ||
| NiO–MnCo2O4 microspheres | 1206 at 200 mA g−1 | 0.01–3 Li/Li+ | 846 (50) at 200 mA g−1 | — | 130 | ||
| MnCo2O4–carbon based composites | 3D sandwich-shaped graphene-based MnCo2O4 hollow spheres | 1244 at 200 mA g−1 | 0.01–3 Li/Li+ | 703 (100) at 200 mA g−1 | 80% (100 cycles) | 131 | |
| Graphene-like 2D spinel MnCo2O4 | 1157.7 at 0.2 A g−1 | 0.01–3 Li/Li+ | 780 (200) at 0.2 A g−1 | — | 139 | ||
| MnCo2O4 nanoparticles embedded in graphene sheets | 1350 at 100 mA g−1 | 0.01–3 Li/Li+ | 584.3 (250) at 2000 mA g−1 | 92.3% (250 cycles) | 138 | ||
| MnCo2O4@carbon cloth | 1886.2 at 1 A g−1 | 0.01–3 Li/Li+ | 1289 (200) at 1 A g−1 | — | 117 | ||
| MnCo2O4/rGO composite | 1657 at 0.1 A g−1 | 0.01–3 Li/Li+ | 791 (100) at 0.2 A g−1 | 74.1% (100 cycles) | 142 | ||
| MnCo2O4/C | 1284.5 at 1 A g−1 | 0.01–3 Li/Li+ | 978 (800) at 1 A g−1 | — | 148 | ||
| MWCNT composite | 1471 at 946 mA g−1 | 0.005–3 Li/Li+ | 871 (30) at 60 mA g−1 | — | 140 | ||
| MnCo2O4-conducting polymer composites | MnCo2O4/polypyrrole | 1398 at 200 mA g−1 | 0.01–3 Li/Li+ | 910 (100)/200 mA g−1 | — | 141 | |
| Flower-like MnCo2O4@PANi–rGO | — | 0.01–3 Li/Li+ | 745 (1050)/500 mA g−1 | — | 143 | ||
| Other MnCo2O4-based composite materials | Mn0.4Ni0.6Co2O4 nanowires | 1054 at 0.1 A g−1 | 0.01–3 Li/Li+ | 706 (200) at 500 mA g−1 | 98% (200 cycles) | 132 | |
| MnCo2O4 porous nanospheres@N-doped carbon | 1099.9 at 1 A g−1 | 0.01–3 Li/Li+ | 883.3 (500) at 1 A g−1 | — | 137 | ||
| Ni-doped MnCo2O4 submicron-spheres | 1849 at 0.2 A g−1 | 0.01–3 Li/Li+ | 174.7 (2000) at 5 A g−1 | — | 133 | ||
| Sodium ion batteries | — | Mesoporous Ni-doped MnCo2O4 hollow nanotubes | 340 at 0.1 A g−1 | 0.01–3 Na/Na+ | 109 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) at 1 A g−1 000) at 1 A g−1 |
81% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
144 |
| — | Flower-like MnCo2O4 | 697 at 25 mA g−1 | 0.01–3 Na/Na+ | 244 (40) at 50 A g-1 | 77.1% (40 cycles) | 145 | |
| MnCo2O4-carbon based composites | MnCo2O4/nanographene | 1120 at 0.05 A g−1 | 0.01–3 Na/Na+ | 541.2 (200) at 0.05 A g−1 | — | 77 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 mA h g−1 at 0.1 mA cm−2. More importantly, after two months of cycling, the microstructure of the cathode was maintained and a recyclability of over 200 cycles was achieved. Other structures with high electrochemical performance can be obtained, such as nanotubes154 (Fig. 5b) and spheres155,156 (Fig. 5c). Composites are also explored to maximize the electrochemical performance of Li–O2 batteries, particularly highlighting materials containing porous carbon,157 Ti4O7
919 mA h g−1 at 0.1 mA cm−2. More importantly, after two months of cycling, the microstructure of the cathode was maintained and a recyclability of over 200 cycles was achieved. Other structures with high electrochemical performance can be obtained, such as nanotubes154 (Fig. 5b) and spheres155,156 (Fig. 5c). Composites are also explored to maximize the electrochemical performance of Li–O2 batteries, particularly highlighting materials containing porous carbon,157 Ti4O7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 and MoO2/Ni
158 and MoO2/Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159.
159.
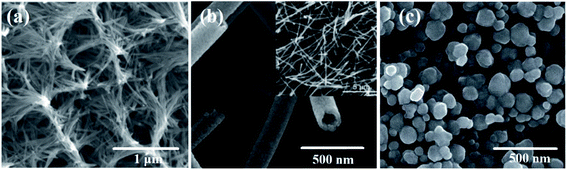 | ||
| Fig. 5 Scanning electron microscopy images (SEM) of different morphologies of MnCo2O4. SEM images of (a) MnCo2O4 nanowires, (b) single-wall MnCo2O4 nanotubes and (c) MnCo2O4 nanospheres. Fig. 1a adapted with permission from ref. 53. Copyright © 2017, American Chemical Society. Fig. 1b, adapted with permission from ref. 154. Copyright © Marketplace™, Royal Society of Chemistry. Fig. 1c adapted with permission from ref. 155. Copyright © Marketplace™, Royal Society of Chemistry. | ||
Large surface areas can provide a promising electrocatalytic activity for the ORR and OER in Li–O2 batteries. In this way, to enhance the charge transfer rate, composites between MnCo2O4 and carbon materials have been reported.152,160–163 A peanut shaped MnCo2O4 which is encapsulated by multi-walled carbon nanotubes (MCO/MWCNTs) was synthesized through a solvothermal method. The batteries exhibited a discharge capacity of 8849 mA h g−1 with a restricted voltage of 2 V at 100 mA g−1 and a cycle life of 120 times at 100 mA g−1 with a limited capacity of 500 mA h g−1.164
Carbonaceous materials and heteroatom doped-carbon materials were also mixed with MnCo2O4 due to their intrinsic advantages as ORR catalysts, such as higher surface area and electrochemical stability. Chandrappa et al.166 reported a composite formed by combining MnCo2O4 nanospheres with graphene sheets (MCO/GS) as a bifunctional cathode catalyst for Zn–air batteries. The electrochemical measurements revealed a unique small charge–discharge overpotential, cycling stability and higher rate capability than a bare MCO catalyst. Carbon coated MnCo2O4 nanowires (MnCo2O4@C) were also used as a bifunctional oxygen catalyst for rechargeable Zn–air batteries. The authors recorded an excellent electrochemical performance and improved cycling stability, with an onset potential of 0.92 V and current retention rate of 99% within 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s at 0.80 V vs. RHE.167 Besides Zn–air batteries,166–169 Na–air devices have also been reported in the literature.170,171Table 3 summarizes the performance of different cells coupled with different metal–air batteries of electrodes based on MnCo2O4.
000 s at 0.80 V vs. RHE.167 Besides Zn–air batteries,166–169 Na–air devices have also been reported in the literature.170,171Table 3 summarizes the performance of different cells coupled with different metal–air batteries of electrodes based on MnCo2O4.
| Type | Cathodes | Initial discharge (mA h g−1) | Discharge voltage (V) | Charge voltage (V) | Overpotentiala (V) | Stability (cycle numbers) | Ref. |
|---|---|---|---|---|---|---|---|
| a The overpotential was calculated based on the difference of discharge–charge voltage plateaus. rGO = reduced graphene oxide, MWCNT = multi wall carbon nanotube, and C = carbon. | |||||||
| Na air battery | Co3O4@MnCo2O4.5 nanocubes | 8400 at 500 mA g−1 | 2.3 | 2.75 | 0.45 | (135 cycles) | 171 |
| dp-MnCo2O4/N-rGO | — | 2.75 | 3.14 | 0.39 | (25 cycles) | 170 | |
| Ni-doped hollow nanotubes | 340.5 at 0.1 A g−1 | — | — | — | 81% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
144 | |
| Zn air battery | MnCo2O4 nanofibers | 125 at 10 mA cm−2 | — | — | 1.23 | (500 cycles) | 168 |
| MnCo2O4 nanoparticles embedded in nitrogen-doped macroporous carbon nanofiber arrays | — | — | — | 0.55 | (100 cycles) | 169 | |
| MnCo2O4@C nanowires | — | — | — | 0.89 | — | 167 | |
| Li air battery | MnCo2O4@Ni | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 at 100 mA g−1 520 at 100 mA g−1 |
2.79 | — | 0.65 | 1000 mA h g−1 (119 cycles) | 150 |
| Nanowires | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 at 0.1 mA cm−2 919 at 0.1 mA cm−2 |
2.92 | 3.46 | 0.54 | 1000 mA h g−1 (144 cycles) | 153 | |
| Microspheres | 2809.1 at 500 mA g−1 | 2.7 | 3.9 | — | 1000 mA h g−1 (50 cycles) | 156 | |
| P-Doped hierarchical porous carbon | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 at 200 mA g−1 150 at 200 mA g−1 |
2.75 | 4.0 | — | 1000 mA h g−1 (200 cycles) | 157 | |
| Ti4O7/MnCo2O4 | 5400 at 100 mA g−1 | 2.85 | 3.6 | 0.75 | 500 mA h g−1 (100 cycles) | 158 | |
| MnCo2O4 nanospheres | 8518 at 100 mA g−1 | 2.86 | — | 0.85 | 1000 mA h g−1 (20 cycles) | 155 | |
| MnCo2O4 nanorods | 1334 at 0.1 mA cm−2 | 2.61 | 4.10 | — | 500 mA h g−1 (40 cycles) | 151 | |
| MnCo2O4/graphene | 3784 at 100 mA g−1 | 2.95 | 3.75 | ∼0.8 | 1000 mA h g−1 (40 cycles) | 162 | |
| Peanut shaped MnCo2O4/MWCNTs | 8849 at 100 mA g−1 | — | — | — | 500 mA h g−1 (120 cycles) | 164 | |
| MnCo2O4/graphene | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092 at 100 mA g−1 092 at 100 mA g−1 |
2.9 | 3.7 | 0.8 | 1000 mA h g−1 (250 cycles) | 161 | |
| MnCo2O4@carbon cloth | 7238 at 200 mA g−1 | — | — | 1.46 | 500 mA h g−1 (108 cycles) | 160 | |
| MnCo2O4–graphene | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092.1 at 200 mA g−1 092.1 at 200 mA g−1 |
— | — | — | 1000 mA h g−1 (35 cycles) | 163 | |
| Double-wall MnCo2O4 nanotubes | 8100 at 100 mA g−1 | 2.77 | 4.14 | 1.37 | 1000 mA h g−1 (278 cycles) | 154 | |
| MnCo2O4/MoO2@Ni nanosheets | 4210 at 200 mA g−1 | — | — | 0.75 | (400 cycles) | 159 | |
| MnCo2O4 nanowires | 8364 at 200 mA g−1 | — | — | 0.823 | 500 mA h g−1 (167 cycles) | 152 | |
4. MnCo2O4-based electrocatalysts for energy conversion and storage
4.1. ORR catalysts in energy storage
As already highlighted in the previous topics, the development of high-performance electrocatalysts is essential for achieving high-performance energy devices, especially catalysts for oxygen reactions (OER and ORR) due to the sluggish reaction kinetics, which often requires a large overpotential to sustain a reasonable rate of electrode reactions.172 In fact, the design and optimization of catalysts for the ORR/OER is of fundamental importance for the development of more efficient and competitive energy storage devices, such as for metal–air batteries173 and proton exchange membrane fuel cells (PEMFCs).174Currently, Ir and/or Ru based oxides and Pt-based materials are the most widely used catalysts for the OER and ORR, respectively. However, the high cost, scarcity, and poor bifunctional activity of precious metals greatly hinder their industrial application on a large scale.175 To solve these disadvantages, intensive efforts have been devoted to development of noble metal-free oxygen reaction catalysts with low cost and high activity in the past decades,176 especially for ORR catalysts. Among these reported noble metal-free ORR catalysts or bifunctional oxygen electrocatalysts, MnCo2O4 and MnCo2O4-derived composites show great potential as electrocatalysts because of their high intrinsic activity, and the corresponding activities can be further tuned through their phase and composition.177
In fact, based on Table 4, it is possible to perceive important and well-known strategies that have been employed in the design of electrocatalysts containing MnCo2O4 for the ORR, as for example: (1) active site engineering, obtained through the control of size, morphology and defects, as well as the crystalline phase, in order to maximize the density of active sites,3,178 and (2) conductivity optimization, obtained especially by doping with hetero-atoms and/or by formation of composites with conducting materials.3,178,179
| ORR catalysts | Incorporated or doping atom | Substrate | E ORR onset potential (V vs. RHE) | Half wave potential (V vs. RHE) | E ORR at −3 mA cm−1 (V vs. RHE) | E OER at 10 mA cm−1 (V vs. RHE) | ΔE EOER–EORR (V vs. RHE) | Current density (mA cm−1) | Tafel slope (mV dec−1) | Average electron transfer number (n) | Stability (h) | pH condition | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a a = obtained by cyclic voltammetry, b = vs. Ag/AgCl, — = information not found in the paper, c = 1 M KOH, d = ΔE = |Ej10 | − |E1/2|, MCO/NS-MCS = cobalt spinel oxides supported on nitrogen and sulfur co-doped mesoporous carbon spheres, N-doped MWCNT = nitrogen-doped multi-walled carbon nanotube, N,S-CNT = N,S-doped carbon nanotubes, 3D-G = three-dimensional graphene, N–C = N-doped carbon, pNGr = N-doped porous graphene, D-AC = AC-based defective carbon, t-CMO CBs = tetragonal CoMn2O4 cubes, tetragonal CoMn2O4 microspheres, and c-CMO NRs = cubic Co2MnO4 nanorods. | |||||||||||||
| MCO-700 | — | GCE | — | — | — | — | — | 6.69 mA cm−1 | — | 3.2–3.5 | −16.66 | 0.1 M KOH | 189 |
| CMO-3.9/CNT | CNT | GCE | — | 0.86 | — | 1.61 | — | 2.90 mA cm−1 at 0.7 V | 65–126 | 3.98 | 94% 8.33 | 0.1 M KOH | 190 |
| MnCo2O4 | — | — | 0.88 | 0.77 | — | — | — | — | — | — | 87% 5.55 | 0.1 M KOH | 167 |
| MnCo2O4@C | Carbon | — | 0.92 | 0.80 | — | 1.66 | 0.89d | — | — | 3.61 | 99% 5.55 | 0.1 M KOH | 167 |
| MnCo2O4/C | Vulcan carbon | GCE | — | 0.76 | — | ∼1.74 | — | — | ∼61 | 3.51 | −8.33 | 0.1 M KOH | 184 |
| MnCo2O4/C nanosheets | Porous C nanosheets | GCE | 0.945 | 0.767 | — | — | — | — | — | 3.82 | 72.5% 2.77 | 0.1 M KOH | 183 |
| MnCo2O4 | — | GCE | ∼0.83a, 1.12 | 0.66 | — | — | — | −2.30 mA cm−1 at 0.36 V | −142.2 | — | −2.22 | 0.1 M KOH | 20 |
| MnCo2O4-rGO | rGO | GCE | ∼0.89a, 1.11 | 0.77 | — | — | — | −3.33 mA cm−1 at 0.36 V | −150.1 | 3.8 | −2.22 | 0.1 M KOH | 20 |
| MCO | — | GCE | −0.165 | −0.225 | −0.647 | 0.840 | 1.487 | — | 145 | n > 3 | — | 0.1 M KOH | 21 |
| MCO + NS-MCS | NS-MCS | GCE | −0.112 | −0.186 | −0.221 | 0.817 | 1.038 | −4.60 mA cm−1 at −1.0 V | 131 | n > 3 | — | 0.1 M KOH | 21 |
| MCO/NS-MCS | NS-MCS | GCE | −0.079 | −0.160 | −0.186 | 0.774 | 0.960 | −5.03 mA cm−1 at −1.0 V | 124 | 3.64–3.88 | 82.3% 5.55 | 0.1 M KOH | 21 |
| MnCo2O4 at 500 °C | — | GCE | 0.81a | 0.58 | — | — | — | — | — | — | 0.1 M KOH | 22 | |
| N-MWCNT-MnCo2O4 at 500 °C | N-MWCNT | GCE | 0.83a, 0.86 | 0.75 | — | — | — | — | — | 3.9 | −20 | 0.1 M KOH | 22 |
| N-MWCNT-MnCo2O4 at 500 °C | N-MWCNT | GCE | 0.86a | 0.60 | — | — | — | — | — | — | — | 0.1 M KOH | 22 |
| MCO | — | GCE | — | — | <0.1 | >1.9 | >2 | — | — | — | — | 0.1 M KOH | 177 |
| MCO + NCNTs | NCNTs | GCE | — | — | 0.70 | 1.74 | 1.04 | — | — | — | — | 0.1 M KOH | 177 |
| MCO@NCNTs | NCNTs | GCE | — | — | 0.76 | 1.70 | 0.94 | — | −96 and −125 | 3.9 | — | 0.1 M KOH | 177 |
| MnCo2O4/CNT | CNT | GCE | — | — | — | — | — | — | — | 3.75 | — | 0.1 M KOH | 191 |
| MnCo2O4/N,S-CNT | N,S-CNT | GCE | — | — | — | — | — | — | — | 3.83 | 72%, 5 | 0.1 M KOH | 191 |
| MnCo2O4/rGO | rGO | GCE | 0.94 | 0.78 | — | — | — | — | 75.4 | 3.90 | — | 0.1 M KOH | 185 |
| MnCo2O4/3D-G | 3D-G | GCE | 0.98 | 0.81 | — | — | — | — | 68.5 | 3.96 | 79.84%, 60 | 0.1 M KOH | 185 |
| MnCo2O4/CNTs | CNTs | GCE | 0.93 | 0.74 | — | — | — | — | 84.6 | 3.81 | — | 0.1 M KOH | 185 |
| MnCo2O4/C | C | GCE | 0.92 | 0.72 | — | — | — | — | 87.4 | 3.76 | — | 0.1 M KOH | 185 |
| MnCo2O4 | — | — | 0.865 | 0.552 | — | — | — | −3.26 mA cm−1 at 0.2 V | — | — | — | 0.1 M KOH | 186 |
| MnCo2O4 + N–C | N–C | — | 0.918 | 0.780 | — | — | — | −5.28 mA cm−1 at 0.2 V | — | — | 0.1 M KOH | 186 | |
| MnCo2O4/N–C | N–C | — | 0.943 | 0.795 | — | — | — | −5.78 mA cm−1 at 0.2 V | 86 | 3.50–3.83 | 89.68% 2.77 | 0.1 M KOH | 186 |
CoMn/pNGr (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
pNGr | GCE | 0.94a, 0.9a | 0.791 | — | — | — | — | 74 | 3.98 | −5000 cycles | 0.1 M KOH | 192 |
CoMn/pNGr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
pNGr | GCE | 0.9a | 0.726 | — | — | — | — | — | 3.66 | — | 0.1 M KOH | 192 |
CoMn/pNGr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
pNGr | GCE | 0.92a | 0.734 | — | — | — | — | — | 3.46 | —, — | 0.1 M KOH | 192 |
| MnCo2O4/NGr | NGr | GCE | 0.86a | 0.648 | — | — | — | — | — | — | —, — | 0.1 M KOH | 192 |
| MnCo2O4/N-rmGO | N-rmGO | GCE | 0.95a, c | — | — | — | — | — | 36c | ∼3.9c | 96.5% 5.55 | 1 M KOH | 188 |
| MnCo2O4 + N-rmGO | N-rmGO mixture | GCE | 0.91a, c | — | — | — | — | — | — | ∼3.7c | ∼75% 5.55 | 1 M KOH | 188 |
| Mesoporous MnCo2O4 | — | GCE | 0.95a | — | 0.80 | 1.63 | 0.83 | — | — | 3.94 | 85% 10 | 0.1 M KOH | 182 |
| m-MnCo2O4 | — | GCE | −0.04 | — | — | — | — | — | −95 | 3.96 | 87% 95 | 0.1 M KOH | 181 |
| MnCo2O4/C | C | GCE | 0.93c | 0.76c | — | 1.52c | 0.59c | — | 68/207c | 3.92c | −24 | 1 M KOH | 193 |
| dp-MnCo2O4/CNT | CNT | GCE | −0.11b | — | — | — | — | −5.33 mA cm−1 at −0.8 Vb | 106 | ∼4.0 | —, — | 0.1 M KOH | 194 |
| dp-MnCo2O4/N-rGO | N-rGO | GCE | −0.09b | — | — | — | — | −5.71 mA cm−1 at −0.8 Vb | 65 | ∼4.0 | —, — | 0.1 M KOH | 194 |
| MnCo2O4 | — | GCE | 0.84 | 0.59 | 0.55 | 1.77 | 1.22 | — | 78.8 | 2.5–3.7 | — | 0.1 M KOH | 187 |
| CMO/20N-rGO | N-rGO | GCE | 0.93 | 0.79 | 0.77 | 1.68 | 0.91 | — | 50.8 | 3.9–4 | 86.3% 8 | 0.1 M KOH | 187 |
| NCNTs | NCNTs | GCE | — | — | — | — | — | — | 3.4 | —, — | 0.1 M KOH | 195 | |
| NCNT-500 | NCNTs | GCE | — | — | — | 1.495 | — | — | — | 3.8 | —, — | 0.1 M KOH | 195 |
| MnCo2O3/C | C | GCE | — | 0.86c | — | — | — | — | 45c | — | —, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
1 M KOH | 196 |
| MnCo2O4/C | C | GCE | — | 0.84c | — | — | — | — | 50c | — | —, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
1 M KOH | 196 |
| c-CMO NRs | — | GCE | 0.9 | 0.72 | — | — | — | 5.9 mA cm−1 | — | 3.9 | 95%, 2.77 | 0.1 M KOH | 180 |
| t-CMO MSs | — | GCE | 0.89 | 0.70 | — | — | — | 5.5 mA cm−1 | — | ∼3.8 | 95%, 2.77 | 0.1 M KOH | 180 |
| t-CMO CBs | — | GCE | 0.89 | 0.65 | — | — | — | 5.51 mA cm−1 | — | 3.6 | 89%, 2.77 | 0.1 M KOH | 180 |
| MnCo2O3/CNF | CNF | GCE | −0.08b | −0.21b | — | — | 1.04 | — | — | ∼3.96 | 89%, 8.33 | 0.1 M KOH | 197 |
| D-AC@2Mn–4Co | D-AC | GCE | 883 | 803 | — | — | — | 4.72 mA cm−1 at 0.2 V | 37.5 | 3.83 | 92.7%, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.1 M KOH | 198 |
| Co2MnO4 | — | — | — | — | 0.59 | 1.92 | 1.33 | — | 3.89 | — | —, — | 0.1 M KOH | 199 |
| Co3O4 + Co2MnO4 | — | — | — | — | 0.55 | 1.86 | 1.31 | — | 3.85 | — | —, — | 0.1 M KOH | 199 |
| Co3O4/Co2MnO4 | — | — | — | — | 0.68 | 1.77 | 1.09 | — | 3.97 | — | ∼94%, 2.77 | 0.1 M KOH | 199 |
As an excellent example of the active site engineering strategy, Yang et al.180 successfully reported a facile precursor pyrolysis method to prepare porous spinel cobalt manganese oxides with tunable size, shape, chemical composition and crystalline structure via a facile precursor pyrolysis method (Fig. 6a). The capping agent and reaction temperature in the reaction were found to be crucial in the formation of porous spinel cobalt manganese oxides from cubic Co2MnO4 nanorods (c-CMO NRs) to tetragonal CoMn2O4 microspheres (t-CMO MSs) and tetragonal CoMn2O4 cubes (t-CMO CBs).
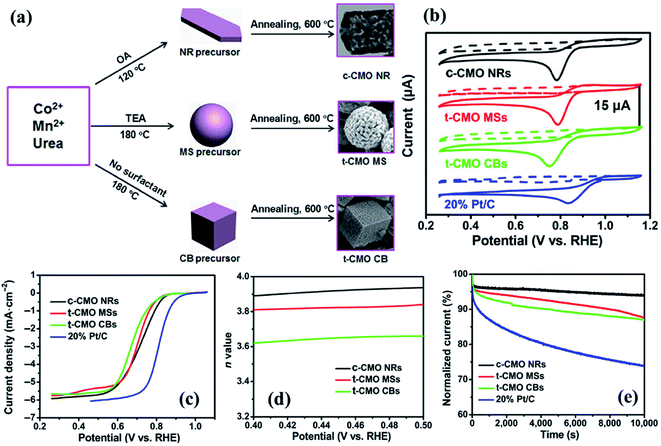 | ||
| Fig. 6 (a) The synthetic process of different spinel CoxMn3−xO4. ORR data of the prepared CMOs in O2versus Ar-saturated 0.1 M KOH with a catalyst mass loading of 0.21 mg cm−2. (b) Cyclic voltammetry curves of electrocatalysts in O2versus Ar-saturated 0.1 M KOH. (c) Linear sweep voltammograms of the electrocatalysts in 0.1 M KOH at 1600 rpm. (d) Electron transfer number n at different potentials. (e) Chronoamperometric responses (percentage of current retained versus operation time) of the different spinel CMOs and 20% Pt/C kept at 0.50 V vs. RHE in O2-saturated 0.1 M KOH. Reproduced with permission from ref. 180. Copyright © 2016, Tsinghua University Press and Springer-Verlag Berlin Heidelberg. | ||
As illustrated in CVs (Fig. 6b) and polarization curves (Fig. 6c) of porous CMOs and 20% Pt/C, all the prepared spinel CMOs exhibited good ORR electrocatalytic activities, of which the c-CMO NRs showed a much more positive onset potential of 0.9 V and a half-wave potential of 0.72 V, which are very close values to those obtained by a commercial Pt/C. Furthermore, the n value of the c-CMO NRs in ORR electrocatalysis was calculated to be about 3.9 in the range of 0.45 and 0.60 V (Fig. 6d, which is in good agreement with a 4-electron oxygen reduction process) and demonstrated a desired durability with negligible degradation of their electrocatalytic activity after a continuous operation time of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seconds (Fig. 6e), which is much better than that of the commercial Pt/C electrocatalyst.180
000 seconds (Fig. 6e), which is much better than that of the commercial Pt/C electrocatalyst.180
The active site engineering strategy has also been used in the development of a mesoporous MnCo2O4 electrode material.181 In one of these studies, Wang and co-workers182 reported a mesoporous bifunctional oxygen MnCo2O4 electrocatalyst synthesized through a spray-pyrolysis route (Fig. 7a), with MnIV in the surface and MnIII in the bulk while CoII was present both in the surface and bulk, as confirmed by X-ray near-edge structure (XANES) and XPS investigation. As a result, the MnCo2O4 exhibited both Co3O4-like activity for the OER (Fig. 7b) and Mn2O3-like performance for the ORR (Fig. 7c), with a potential difference between the ORR and OER of 0.83 V. According to the Koutecky–Levich (K–L) equation, the electron transfer number (n) of MnCo2O4 was calculated to be 3.94 and after 10 h, the loss of current density for MnCo2O4 was only 15%, indicating higher stability of MnCo2O4 than Pt/C (Fig. 7e). Another advantage is that the electrode material can be obtained on a large-scale at a relatively low temperature with precise chemical control of the components. The prominent bifunctional activity shows that MnCo2O4 could be used in metal–air batteries and/or other energy devices, as confirmed by the home-build Zn–air battery used to study the bifunctional stability of mesoporous MnCo2O4 (Fig. 7e).182
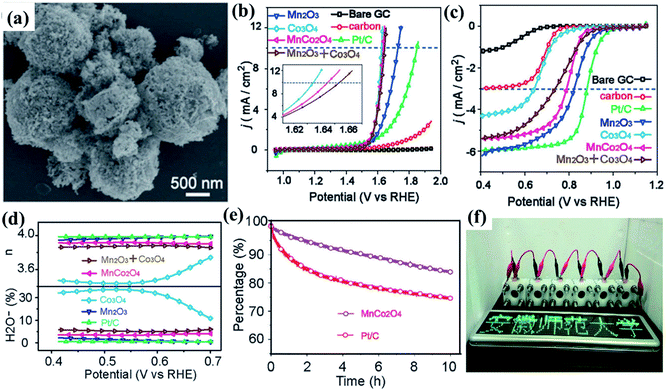 | ||
| Fig. 7 (a) SEM of MnCo2O4; (b) OER polarization curves of catalysts at 1600 rpm; (c) ORR polarization curves of catalysts at 1600 rpm; (d) percentage of peroxide and electron numbers (n) of Co3O4, Mn2O3, MnCo2O4, Pt/C, and the physical mixture of Mn2O3 and Co3O4. (e) Chronoamperometric measurements of MnCo2O4 and Pt/C at −0.3 V (V vs. Ag/AgCl) in O2-saturated 0.1 M KOH at 1600 rpm. (f) A green light emitting diode (LED) panel powered by six Zn–air batteries (containing MnCo2O4). Reproduced with permission from ref. 182. Copyright © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim University Press and Springer-Verlag Berlin Heidelberg. | ||
On the other hand, conductivity optimization by the formation of composites with conducting carbon materials has been the main strategy for preparing excellent electrocatalysts for the ORR. In fact, pure MnCo2O4 nanoparticles displayed certain ORR catalytic activity, but with a poor onset potential and peak potential.183 In this context, among the most used carbon materials for this application, it is possible to highlight Vulcan carbons,184 CNTs22 and graphene derivatives.20
In one of these studies, Zhang et al.185 designed a strategy to prepare MnCo2O4 on three-dimensional graphene (3D-G), as shown in Fig. 8. Typically, 3D-G (with multilayered structure of graphene) was synthesized using a coal tar pitch as the carbon source and nano MgO as the template (Fig. 8a). Then, spinel MnCo2O4 nanoparticles were in situ prepared and deposited on the inner walls of pores in the 3D-G by a facile hydrothermal method, resulting in the MnCo2O4/3D-G composite.185 Surprisingly, the MnCo2O4/3D-G catalyst showed an onset potential of 0.98 V (vs. RHE) and the half-wave potential was 0.81 V (vs. RHE) in a solution of 0.1 M KOH (Fig. 8b), which was clearly superior to those of 20 wt% Pt/C (0.97 V, 0.80 V), MnCo2O4/rGO (0.94 V, 0.78 V), MnCo2O4/CNTs (0.93 V, 0.74 V), and MnCo2O4/C (0.92 V, 0.72 V).185 In addition, the electron transfer number was 3.96 at 0.4 V (vs. RHE), and its catalyzed ORR mainly follows a four-electron process (Fig. 8c), indicating that the MnCo2O4/3D-G catalyst possesses superior selectivity for the ORR process. Besides that, MnCo2O4/3D-G showed the lowest Tafel slope of 68.5 mV dec−1 compared to those of Pt/C (70.2 mV dec−1 of 20 wt%), MnCo2O4/rGO (75.4 mV dec−1), MnCo2O4/CNTs (84.6 mV dec−1) and MnCo2O4/C (87.4 mV dec−1) (Fig. 8d), and the durability test demonstrated that the MnCo2O4/3D-G catalyst has a much better durability than commercial Pt/C.185 This work shows that the preparation of composites with carbonaceous materials is really an inspiring strategy to prepare high performance electrocatalysts for the development of fuel cells.
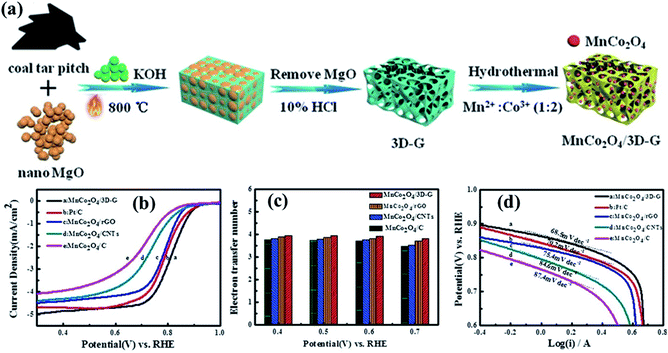 | ||
| Fig. 8 (a) The synthesis route to the MnCo2O4/3D-G catalyst. (b) LSV curves of the different catalysts. (c) Electron transfer number per oxygen molecule of the different catalysts at different potentials. (d) Tafel slopes of the electrode assemblies fabricated with different catalysts. Reproduced with permission from ref. 185. Copyright © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
It is important to highlight that results as promising as those obtained in the work by Zhang et al.185 were obtained by forming composites (conductivity optimization strategy) with conductive carbonaceous materials, applying the active site engineering strategy to the catalytic nanocarbon sites of these composites. In this combination of strategies, N and S-doped carbonaceous materials have been intensively studied,21 for example, Fu and co-workers186 successfully prepared composed of N-doped carbon (N–C) and MnCo2O4 NPs for the ORR (MnCo2O4/N–C), with an ORR onset potential of 0.943 V, ORR half-wave potential of 0.795 V, synthesized by pyrolyzing the mesoporous-silica-protected zeolitic imidazolate framework-8 (ZIF-8) and etching, followed by a facile hydrothermal procedure (Fig. 9a). The superior performance of MnCo2O4/N–C was attributed to its porous structure and large surface area, N-doping effect, small size MnCo2O4 NPs and synergistic effects between the doped active species.186 Based on the values of onset and half-wave potentials shown in Table 1, it is possible to infer that the MnCo2O4/N–C reported by Fu et al.186 presented slightly better performance than other composites containing N-doped carbonaceous materials, such as N-MWCNT-MnCo2O4 (ORR onset potential of 0.86 V, ORR half-wave potential of 0.75 V),22 and CMO/20N-rGO (ORR onset potential of 0.93 V, ORR half-wave potential of 0.79 V).187
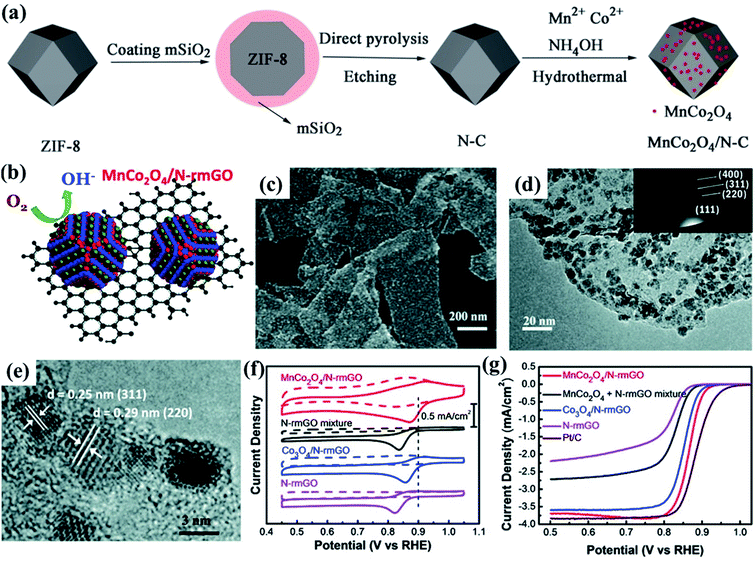 | ||
| Fig. 9 Schematic illustration of preparation of (a) MnCo2O4/N–C nanocomposites. Reproduced with permission from ref. 186. Copyright © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Schematic illustration of preparation of (b) the MnCo2O4/N-rmGO hybrid. (c) SEM image and (d) TEM image with an inset of the electron diffraction pattern of the MnCo2O4/N-rmGO hybrid, respectively. (e) A high-magnification TEM image of the MnCo2O4/N-rmGO hybrid. (f) CV curves of the MnCo2O4/N-rmGO hybrid, MnCo2O4 + N-rmGO mixture, Co3O4/N-rmGO hybrid, and N-rmGO on glassy carbon electrodes in O2-saturated (solid line) or N2-saturated (dashed line) 1 M KOH. The peak position of Pt/C was shown as a dashed line for comparison. (g) Rotating-disk electrode voltammograms of the MnCo2O4/N-rmGO hybrid, MnCo2O4 + N-rmGO mixture, Co3O4/N-rmGO hybrid, N-rmGO, and Pt/C in O2-saturated 1 M KOH at a sweep rate of 5 mV s−1 at 1600 rpm. Reproduced with permission from ref. 188. Copyright © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Using a slightly different approach, Liang et al.188 developed hybrid composites through direct NP nucleation and growth on nitrogen doped-reduced graphene oxide (N-rmGO) sheets and Mn substitution of spinel Co3O4 NPs (average size of ∼5 nm) for the ORR under alkaline conditions (Fig. 9b), as confirmed by the SEM (Fig. 9c) and TEM (Fig. 9d) images, as well as by the HRTEM images, showing the lattice fringes of the nanocrystals, consistent with the MnCo2O4 crystal structure (Fig. 9e). This method results in covalent coupling between oxide NPs and N-rmGO sheets, yielding higher activity and stronger durability than the physical mixture of NPs and N-rmGO.188
Interestingly, the C–O and C–N bonds in the N-rmGO sheet were strongly perturbed, suggesting the formation of C–O–metal and C–N–metal bonds between N-rmGO and spinel oxide NPs, as confirmed by electrochemical and XANES investigations. As a result, the composite showed a more positive onset (0.95 V vs. RHE, Fig. 9f–g) and a greater electron transfer number (∼3.9) than the corresponding physical mixture of MnCo2O4 NPs and N-rmGO (0.91 V vs. RHE and the electron transfer number = ∼3.7).188 Based on the above, it is feasible to mention that the combination of conductivity optimization and active site engineering strategies should benefit the design of advanced ORR electrocatalysts for energy conversion and storage.
4.2. Water-splitting electrocatalysts for energy conversion (OER and HER)
Over the years, the energy demand has increased significantly, and this consumption has intensified year after year, which has led to the depletion of non-renewable energy sources (fossil fuels), as well as an increase in environmental pollution.200 Therefore, the development of new technologies, in which energy is obtained safely, cheaply, and without harming the environment is essential for the preservation of our society.Among the energy conversion systems, electrochemical water splitting has proved to be very efficient when it comes to obtaining clean and high purity fuels.201 In fact, from the electrochemical water splitting it is possible to obtain O2 and H2 through OER and HER that occur at the anode and cathode electrodes, respectively.2,202
The HER can be expressed depending on electrolyte pH, according to eqn (1) and (2):
| Acidic medium: 2H+ + 2e− → H2 | (1) |
| Neutral/alkaline medium: 4H2O + 4e− → 2H2 + 4OH− | (2) |
In addition, the HER can be divided into two steps called Volmer and Heyrovsky or Tafel pathways, where Hads acts as an intermediate species and plays a crucial role in the mechanism. The first step can proceed in acidic (eqn (3)) and neutral/alkaline solutions (eqn (4)), as indicated by the equations below:
| H+ + e− → Hads | (3) |
| H2O + e− → Hads + OH− | (4) |
Depending on the coverage ratio of Hads (θH), the second step can occur through Heyrovsky or Tafel pathways. Whereas the Heyrovsky pathway occurs due to low θH (eqn (5) in acidic medium and eqn (6) in neutral/alkaline medium), the Tafel pathway occurs in consequence of high θH, regardless of the pH value (eqn (7)).
| Hads + H+ + e− → H2 | (5) |
| H2O + e− + Hads → H2 + OH− | (6) |
| Hads + Hads → H2 | (7) |
However, the obtaining of H2 is limited by the sluggish reaction kinetics of the OER, because of the four-step electron transfer process (4OH− → H2O + O2 + 4e−) in neutral or alkaline medium.
Currently, catalysts formed by using noble metals, such as RuO2, IrO2, and Pt, have been used in electrochemical water splitting in order to overcome the slow reaction kinetics of the OER.203 However, these catalysts are scarce and expensive, and their use in industry is not feasible. Therefore, noble metal catalysts have been replaced by alternative ones such as layered double hydroxides,2,204 oxides,205,206 nitrides and sulfides,207,208 and spinel structures.209,210 Among these catalyst groups, spinel oxides with AB2O4 (A and B transition metals) in special MnCo2O4 have stood out as promising electrode materials for water splitting due to the ease of preparation, variable valence states and high redox stability in alkaline medium.194
The main MnCo2O4-based catalysts for the OER and HER are shown in Table 5. Most of the studies reported in the literature are related to the electrochemical performance of MnCo2O4 concerning the OER, and very few studies were found in the literature using MnCo2O4 as an electrocatalyst for the HER, suggesting that there is a vast unexplored field that deserves attention.
| Catalysts | Substrate | Overpotential at 10 mA cm−2 (mV vs. RHE) | Onset potential (V vs. RHE) | Tafel slope (mV dec−1) | Stability (h) | pH condition | Ref. | |
|---|---|---|---|---|---|---|---|---|
| a NW = nanowire; NF = nickel foam; NS = nanosheet; MCO = MnCo2O4; NS-MCS = nitrogen and sulfur co-doped mesoporous carbon spheres; rGO = reduced graphene oxide; GCE = glassy carbon electrode; YSM = yolk–shell; N-rmGO = N-doped reduced graphene oxide; NC = nitrogen doped carbon; CMO/20N-rGO = Co3O4–MnCo2O4/N-doped reduced graphene oxide with a mass ratio of NrGO/(Co + Mn) of ca. 20 wt%; NFF = Ni–Fe foam. | ||||||||
| OER catalysts | MnCo2O4@CoS NW | NF | 280 at 20 mA cm−2 | 1.51 | 139.19 | 10 | 1.0 M KOH | 217 |
| MnCo2O4@CoS NS | NF | 270 at 20 mA cm−2 | 1.50 | 131.81 | 10 | 1.0 M KOH | 217 | |
| MCO/NS-MCS | NS-MCS | 508 | 1.738 | 124 | 5.5 | 0.1 M KOH | 21 | |
| MnCo2O4 | — | 290 | 1.52 | 97 | 24 | 1.0 M KOH | 193 | |
| MnCo2O4@Mn–Co–P | Ti | 269 | 1.50 | 102 | 100 | 1.0 M KOH | 224 | |
| Mn1−xNixCo2O4/rGO | CGE | 250 | 1.48 | 78 | 2.8 | 1.0 M KOH | 211 | |
| Mn1−xZnxCo2O4/rGO | GCE | 320 | 1.48 | 80.6 | 2.8 | 1.0 M KOH | 212 | |
| YSM-MCO | NF | 360 | 1.59 | 65.6 | 80 | 1.0 M KOH | 225 | |
| MnCo2O4@Ni3S2 | NF | 200 at 40 mA cm−2 | — | 43.9 | 15 | 1.0 M KOH | 226 | |
| MnCo2O4/N-rmGO | GCE | 330 | 1.56 | — | — | 1.0 M KOH | 188 | |
| MnCo2O4-rGO | GCE | 530 | 1.56 | 106.9 | — | 0.1 M KOH | 20 | |
| MnxCo3−xO4 | NF | 327 | — | 79 | 25 | 1.0 M KOH | 227 | |
| MnCo2O4@Ni2P | NF | 240 | — | 114 | 30 | 1.0 M KOH | 221 | |
| MnCo2O4@NC | GCE | 287 | 1.46 | 55 | 20 | 0.1 M KOH | 218 | |
| Ce–MnCo2O4 | GCE | 390 | — | 125 | 11.1 | 1.0 M KOH | 213 | |
| CMO/20N-rGO | GCE | 450 | 1.68 | 80.2 | 8 | 0.1 M KOH | 187 | |
| Ce–MnCo2O4 | GCE | 389 | — | 96 | 12 | 1.0 M KOH | 213 | |
| HER catalysts | MnCo2O4@Ni2P | NF | 57 | — | 89 | 20 | 1.0 M KOH | 221 |
| NiFe–MnCo2O4/NFF | NFF | 98 | — | 80.78 | 48 | 1.0 M KOH | 19 | |
| MnCo2O4@Ni3S2 | NF | 110 | — | 212.15 | 15 | 1.0 M KOH | 226 | |
Despite advantages mentioned above, the catalytic activity of MnCo2O4 is limited by its low electrical conductivity. However, strategies have been explored in order to improve the electrical conductivity such as introduction of hetero-atoms, combining MnCo2O4 with conducting materials forming composites, incorporation of oxygen vacancies and nanoparticle size control.
In this sense, Rebekah and co-authors211,212 showed that the catalytic activity of MnCo2O4 has been improved by the introduction of hetero-atoms (Ni and Zn), as well as by the combination of spinel oxide with rGO. Both Ni and Zn substituted MnCo2O4 on the rGO surface were synthesized through a hydrothermal method. The electrochemical behavior of Mn1−xNixCo2O4/rGO and Mn1−xZnxCo2O4/rGO electrodes towards the OER was verified by Linear Sweep Voltammetry (LSV). Better results were achieved for the following compositions, Mn0.4Ni0.6Co2O4/rGO211 (overpotential of 250 mV at 10 mA cm−2 and a Tafel slope of 78 mV dec−1) and Mn0.8Zn0.2Co2O4/rGO212 (overpotential of 320 mV at 10 mA cm−2 and a Tafel slope of 80.6 mV dec−1). In summary, good electrochemical performance achieved by Mn1−xNixCo2O4/rGO and Mn1−xZnxCo2O4/rGO electrodes towards the OER can be explained by the faster electron transport due to the more exposed active sites in consequence of the high surface area of rGO and of the reduction of metal ion aggregation owing to stacking between the sheets. In addition, the incorporation of another metallic ion resulted in a material with excellent electrochemical behavior and high conductivity.
The strategy of doping MnCo2O4 was also used by Huang et al.213 in order to obtain an efficient electrocatalyst for both OER and HER. Indeed, the authors doped MnCo2O4 with Ce, being named as Ce–MnCo2O4. The OER and HER performances were evaluated by LSV and the material reached an overpotential of 390 and 379 mV, respectively. Those values when compared to MnCo2O4 without Ce doping are much superior. In fact, MnCo2O4 without Ce presented an overpotential at 10 mA cm−2 of 560 and 477 mV, respectively for the OER and HER. The OER results can be attributed to the introduction of Ce into MnCo2O4 that facilitates oxygen transfer through adsorption, dissociation and release of atomic O for the OER, besides the introduction of oxygen vacancies to dissociate water.214–216
Also, the electrical conductivity of MnCo2O4 can be improved through design of hierarchical 3D core@shell structures. Indeed, Du and co-authors217 reported the synthesis of two materials based on MnCo2O4@CoS with different morphologies, using a hydrothermal method followed by the electrodeposition technique. As a matter of fact, MnCo2O4@CoS was synthesized in the nanowire and nanosheet shapes, as can be seen in TEM images displayed in Fig. 10a and b, respectively.
 | ||
| Fig. 10 TEM images of (a) MnCo2O4@CoS nanowires and (b) MnCo2O4@CoS nanosheets. Reproduced with permission from ref. 217. Copyright © 2019 Hydrogen Energy Publications LLC. Published by Elsevier Ltd. All rights reserved. (c, d) TEM and (e) HRTEM images of MnCo2O4@NC. (f) LSV curves of MnCo2O4@NC, RuO2 and MnCo2O4 and (g) LSV initial curve and after 4000 curves for MnCo2O4@NC. Reproduced with permission from ref. 218. Copyright © 2016, Tsinghua University Press and Springer-Verlag Berlin Heidelberg. | ||
The electrocatalytic activity performances of MnCo2O4@CoS with different morphologies towards the OER are very similar, reaching 280 mV and 270 mV at 20 mA cm−2 for MnCo2O4@CoS nanowires and MnCo2O4@CoS nanosheets, respectively. Also, the surface areas of the materials were very similar as well. For MnCo2O4@CoS nanowires and MnCo2O4@CoS nanosheets the BET surface areas were 68.46 and 69.38 m2 g−1, respectively. Therefore, the electrochemical results cannot be attributed to the materials' surface area, but it can be related to the synergistic effect between MnCo2O4 and CoS, since the poor electrical conductivity of MnCo2O4 is compensated by the conductive CoS, and by the abundant oxygen vacancies. In fact, the existence of a large amount of oxygen vacancies can be estimated through XPS studies, where due to the co-existence of Co2+ and Co3+ ions the molar ratio of Co2+/Co3+ is a good parameter to evaluate the oxygen vacancies. The molar ratio found for the MnCo2O4@CoS nanowires and MnCo2O4@CoS nanosheets was 0.88 and 0.93, respectively, indicating a large amount of oxygen vacancies.
Over the years, several studies have been reported in the literature where it is demonstrated that the size control of nanoparticles plays a key role in improving the properties of the materials. Using this strategy, Su et al.218 encapsulated MnCo2O4 nanoparticles using nitrogen-doped carbon (NC), since the NC can not only act as an encapsulating agent controlling the growth of the nanoparticles but also improve the catalytic performance in water splitting.219 The MnCo2O4 was encapsulated in NC, here denoted as MnCo2O4@NC from a metal–organic complex as a precursor, using a hydrothermal method. The TEM and HRTEM images of the as-prepared material are shown in Fig. 10c–e. It is possible to observe that the MnCo2O4@NC nanowires were made of several well-dispersed nanoparticles with less than 10 nm of average diameter (Fig. 10c). Besides, in Fig. 10e, the HRTEM image of MnCo2O4@NC is displayed, where it is clearly possible to observe that MnCo2O4 was encapsulated by NC, forming a core@shell structure. However, the MnCo2O4 nanowires without NC presented nanoparticles with an average diameter of 100 nm, demonstrating that the growth of MnCo2O4 nanoparticles was limited by the NC.
The results presented above can directly influence the catalytic activity of the material. The MnCo2O4@NC presented an electrochemical performance (overpotential of 287 mV at 10 mV cm−2 and Tafel slope of 55 mV dec−1) far superior to that of MnCo2O4 nanowires (overpotential of ∼420 mV at 10 mV cm−2 and Tafel slope of 101 mV dec−1), Fig. 10f. Also, the MnCo2O4@NC did not show any change in the electrochemical profile after 4000 cycles, as can be seen in Fig. 10g. These results demonstrated that the size control of MnCo2O4 nanoparticles (less than 10 nm) using NP, in order to limit the growth of the nanoparticles, provided several active sites are exposed to oxygen adsorption and desorption. Besides, the nanoporous core@shell structure provided easy access of electrolyte ions and improved the electron transfer rate.
Recent studies have pointed out that one of the causes of the low catalytic activity and stability of Co-based compounds for water oxidation is the Jahn–Teller distortion. In this sense, Hirai et al.220 performed a systematic study for tetragonal spinel oxides Mn3−xCoxO4 (0 ≤ x < 1 and 1 < x ≤ 1.5) in order to evaluate the relation between the Jahn–Teller distortion and catalytic activity for the OER. They figured out that for Mn3−xCoxO4 (0 ≤ x < 1) the catalytic activity was improved with the increase of Co concentration, due to the Jahn–Teller distortion suppression. However, for Mn3−xCoxO4 (1 < x ≤ 1.5) the OER activity decreased with Co concentration above 1 up to 1.5, when compared to Mn3−xCoxO4 (0 ≤ x < 1), as can be seen through LSV curves in Fig. 11a. Although Mn3+ still remains occupying the octahedral sites on the Mn3−xCoxO4 (0 ≤ x < 1), when cobalt is added it will occupy the tetrahedral sites, and thus the Jahn–Teller distortion is suppressed, represented by an indicator c/√2a (Fig. 11b) and consequently there is an increase in catalytic activity, due to the strong interaction between the antibonding electron eg and oxygen species adsorbed (O22− and O2−). Nevertheless, when the cobalt concentration is above >1, the octahedral sites are occupied by a mixture of Mn3+, Co3+, Mn4+, and Co2+ ions and then the eg orbital can be occupied by more than 1 electron or even not be occupied thus decreasing the catalytic activity.
 | ||
| Fig. 11 LSV curves of different samples of Mn3−xCoxO4 (0 < x ≤ 1.5) (a) and schematic orbital energy diagram for Mn3+ 3d at octahedral sites and Mn2+ and Co2+ at tetrahedral sites (b). Reproduced with permission from ref. 220. Copyright © Marketplace™, Royal Society of Chemistry. TEM image of MnCo2O4@Ni2P (c), LSV curves for the electrocatalytic performance of the OER (d) and HER (e). Reproduced with permission from ref. 221. Copyright © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Similarly, Ge et al.,221 using an interface engineering strategy to suppress the Jahn–Teller distortion in MnCo2O4, grew Ni2P nanosheets on the MnCo2O4 surface obtaining a bifunctional catalyst for the OER and HER. The MnCo2O4@Ni2P was obtained in four steps. The precursor MnCo-LDH was obtained through the hydrothermal method, and then the as-precursor MnCo-LDH was annealed in order to obtain MnCo2O4. A second hydrothermal method was used to deposit Ni(OH)2 nanosheets on MnCo2O4. After that, the MnCo2O4@Ni(OH)2 was calcined in the presence of NaH2PO2 in order to obtain MnCo2O4@Ni2P. In Fig. 11c the TEM image of MnCo2O4@Ni2P is displayed, where it is possible to observe that the MnCo2O4 nanoneedles are coated by a large number of Ni2P nanosheets.
The electrochemical performance of MnCo2O4@Ni2P towards the OER and HER was evaluated by LSV, and the curves are shown in Fig. 11d and e. The hierarchical MnCo2O4@Ni2P structure exhibited an excellent overpotential for the OER (240 mV at 10 mA cm−2) and a Tafel slope of 114 mV dec−1. Also, the MnCo2O4@Ni2P showed an outstanding HER performance with an overpotential of 10 mA cm−2 (57 mV) and a Tafel slope of 89 mV dec−1. This HER performance can be explained by the presence of several Ni0 and Ni2+ species, as determined by XPS after phosphorization, once those species can provide energy in order to stabilize the Hads through the weakening of the O–H bond of adsorbed water.222
The electronic interactions between MnCo2O4 and Ni2P were clarified through differential charge density. The high charge density is placed on the Ni2P side, and thus the electrons migrate from Ni2P to MnCo2O4 due to the strong interfacial polarization.223 Thus, the strong interaction between MnCo2O4@Ni2P reduces the Jahn–Teller distortion and the Ni2P with metallic properties increases the electronic conductivity and charge transfer rate of MnCo2O4@Ni2P.
5. Conclusions and future directions
Lately spinel MnCo2O4-based materials have stood out in the energy conversion and storage technologies, especially due to their low cost, simple preparation and chemical composition versatility obtained through different strategies that enabled the rational design of these materials, thus allowing tuning of their electronic properties. In addition, reducing Co ions in the structure of Co3O4 by replacing them with Mn ions has been an excellent strategy to increase the conductivity and improve the electrochemical performance of the electrode materials. In fact, studies have shown that the conductivity of MnCo2O4 is greater than that of Co3O4, and its electrochemical performance is superior since Co provides a higher oxidation potential than Mn and Mn brings a higher capacity than Co owing to its efficient electron transport.115 Thus, the recent advances have been summarized in this review and special emphasis was directed to spinel MnCo2O4 based materials, which are highly promising for the construction of supercapacitors and batteries, and thus the development of arrangements for water-splitting for energy conversion. It is necessary to highlight that the application of MnCo2O4 as a multifunctional material still needs fine control of synthesis conditions, which will impact the phase, morphology, cation distribution, and especially the electrical properties, and it is an important requirement for energy applications.The construction of supercapacitors using MnCo2O4 in the pristine form or as composites based on metal oxides/hydroxides, polymers or carbon-based composites or other materials was described as an important strategy to increase the capacitance and rate capability of hybrid devices, resulting in supercapacitors with higher energy and power density.
Additionally, LIBs, SIBs and metal–O2 batteries are also covered. In order to improve the electrochemical performance in LIBs, in addition to developing composites of MnCo2O4 with other materials, morphological modifications and synthesis methods have been the focus of researchers, particularly on the preparation of porous materials (mesoporous, hollow, yolk–shell, shell-in-shell, and yolk-in-double-shell spheres). Mesoporous transition metal-doped MnCo2O4 has also been reported for application in SIBs, while heteroatoms doped-carbon materials were mixed with MnCo2O4 due to intrinsic advantages as ORR catalysts, such as high surface area and electrochemical stability in metal–O2 batteries. The results obtained with MnCo2O4 for the construction of supercapacitors, metal-ion batteries, and metal–air batteries were tabulated.
Conversely, MnCo2O4 based materials showed great potential as electrocatalysts in energy storage devices. For instance, conductivity optimization by the formation of composites with conducting carbon materials has been the main strategy for preparing excellent electrocatalysts for the ORR (or bifunctional electrocatalysts for the OER/ORR). However, porous spinels with tunable size, shape, chemical composition and crystalline structure were also reported via a facile precursor pyrolysis method.
MnCo2O4 was also recently used as an electrocatalyst for energy conversion, mainly in water splitting. Most of the studies reported in the literature are related to the electrochemical performance of MnCo2O4 concerning the OER, and very few studies were found in the literature using MnCo2O4 as an electrocatalyst for the HER, suggesting that there is a vast unexplored field that deserves attention. Thereunto, strategies have been explored in order to improve the electrical conductivity and electrocatalytic activity, such as introduction of hetero-atoms (or doping), combination with conducting materials forming composites, incorporation of oxygen vacancies and nanoparticle size control.
As demonstrated in this article, the utilization of spinel MnCo2O4-based materials for energy storage and conversion is a promising new concept in energy technologies. It is also important to highlight that future research should continue to enrich spinel MnCo2O4-based materials, focusing attention on the electrochemical performance and reasonable architectural design of those materials for practical application in energy technologies and expanding the fields of application. In fact, it is possible to visualize a field still unexplored and of great potential for application, for instance, the rational design of different composite materials composed of MnCo2O4 and/or heterojunctions containing the recent advanced 2D materials (such as MXene, transition metal chalcogenides, black phosphorus, etc.), aiming at future applications of these supercapacitor/battery materials for flexible/wearable devices, self-charged energy storage devices, and microsupercapacitors. The future applications of flexible/wearable energy devices depend on suitable flexible substrates that can stably and efficiently incorporate MnCo2O4 or its composites. In this direction, novel current collectors have been investigated and carbon cloth has shown promising results due to its excellent flexibility and conductivity. Additionally, different synthesis strategies urge investigation, and in this sense, electrochemical growth on flexible conductive substrates is promising (without binders and using a simplified rotocol). Considering large-scale and reproducible production, additive manufacturing (or three-dimensional printing) protocols offer great promise in this area.
Moreover, bifunctional and multifunctional catalysts represent promising directions,15 especially for electrocatalysts for water splitting (HER/OER) and metal–air batteries (ORR/OER). Other applications, such as the use of MnCo2O4 catalysts to convert greenhouse gases (CO2) and toxic gas (CO) into chemical fuels, are also feasible. From this perspective, MnCo2O4-based materials have a key role in the way to a more sustainable society and industrial applications.
Conflicts of interest
The authors declare that there is no conflict of interest.Acknowledgements
This work was supported by the Sao Paulo Research Foundation (FAPESP processes 2018/16896-7, 2017/13137-5 and 2020/00325-0), the National Council for Scientific and Technological Development (CNPq Processes 307271/2017-0 and 311847/2018-8), Coordination for the Improvement of Higher Education Personnel (CAPES) Financial code 001 and to Brazilian Institute of Science and Technology in Bioanalytics (INCTBio) (CNPq grant no. 465389/2014-7 and FAPESP grant no. 2014/50867-3) and Brazilian Institute of Science and Technology (INCT) in Carbon Nanomaterials. CSR acknowledges the Department of Science and Technology (DST)-SERB Early Career Research project (Grant No. ECR/2017/001850), DST-Nanomission (DST/NM/NT/2019/205(G)) and Karnataka Science and Technology Promotion Society (KSTePS/VGST-RGS-F/2018-19/GRD NO. 829/315).References
- S. Ghosh and R. N. Basu, Nanoscale, 2018, 10, 11241–11280 RSC
.
- J. M. Gonçalves, P. R. Martins, L. Angnes and K. Araki, New J. Chem., 2020, 44, 9981–9997 RSC
.
-
J. M. Gonçalves, T. A. Matias, K. C. F. Toledo and K. Araki, in Advances in Inorganic Chemistry, ed. R. V. Eldik and C. Hubbard, Elsevier, 2019, vol. 74, p. 63 Search PubMed
.
- J. Xu, C. Chen, Z. Han, Y. Yang, J. Li and Q. Deng, Nanomaterials, 2019, 9, 1161 CrossRef CAS
.
- J. M. Gonçalves, M. I. da Silva, H. E. Toma, L. Angnes, P. R. Martins and K. Araki, J. Mater. Chem. A, 2020, 8, 10534–10570 RSC
.
- S. J. Uke, V. P. Akhare, D. R. Bambole, A. B. Bodade and G. N. Chaudhari, Front. Mater., 2017, 4, 21 Search PubMed
.
- X. Wang, A. Hu, C. Meng, C. Wu, S. Yang and X. Hong, Molecules, 2020, 25, 269 CrossRef CAS
.
- Y. Shi, X. Pan, B. Li, M. Zhao and H. Pang, Chem. Eng. J., 2018, 343, 427–446 CrossRef CAS
.
- X. Hu, L. Wei, R. Chen, Q. Wu and J. Li, ChemistrySelect, 2020, 5, 5268–5288 CrossRef CAS
.
- M. Hamdani, R. N. Singh and P. Chartier, Int. J. Electrochem. Sci., 2010, 5, 556–577 CAS
.
- J. Zhu, Q. Wu and J. Li, ChemistrySelect, 2020, 5, 10407–10423 CrossRef CAS
.
- A. U. Ubale, M. A. Waghmare, K. S. Iqbal and H. M. Pathan, J. Mater. Sci.: Mater. Electron., 2020, 31, 14003–14021 CrossRef CAS
.
- L. Tian, X. Zhai, X. Wang, J. Li and Z. Li, J. Mater. Chem. A, 2020, 8, 14400–14414 RSC
.
- X. Zhao, L. Mao, Q. Cheng, J. Li, F. Liao, G. Yang, L. Xie, C. Zhao and L. Chen, Chem. Eng. J., 2020, 387, 124081 CrossRef CAS
.
- Y. Li, X. Han, T. Yi, Y. He and X. Li, J. Energy Chem., 2019, 31, 54–78 Search PubMed
.
- J. P. Cheng, W. D. Wang, X. C. Wang and F. Liu, Chem. Eng. J., 2020, 393, 124747 CrossRef CAS
.
- X. Han, X. Gui, T.-F. Yi, Y. Li and C. Yue, Curr. Opin. Solid State Mater. Sci., 2018, 22, 109–126 CrossRef CAS
.
- R. Kumar, Nano-Micro Lett., 2020, 12, 122 CrossRef CAS
.
- Y. Lin, Z. Yang, D. Cao and Y. Gong, Crystengcomm, 2020, 22, 1425–1435 RSC
.
- H. Yang, M. Zhu, X. Guo, C. Yan and S. Lin, ACS Omega, 2019, 4, 22325–22331 CrossRef CAS
.
- T. Oh, S. Ryu, H. Oh and J. Kim, Dalton Trans., 2019, 48, 945–953 RSC
.
- S. Yuvaraj, A. Vignesh, S. Shanmugam and R. Kalai Selvan, Int. J. Hydrogen Energy, 2016, 41, 15199–15207 CrossRef CAS
.
- Y. Li, M. S. Wu and C. Y. Ouyang, Appl. Surf. Sci., 2015, 349, 510–515 CrossRef CAS
.
- N. R. Chodankar, H. D. Pham, A. K. Nanjundan, J. F. S. Fernando, K. Jayaramulu, D. Golberg, Y.-K. Han and D. P. Dubal, Small, 2020, 16, 2002806 CAS
.
- Y. Gogotsi and R. M. Penner, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS
.
- V. Sannasi and K. Subbian, Ceram. Int., 2020, 46, 15510–15520 CrossRef CAS
.
- S. G. Krishnan, M. H. A. Rahim and R. Jose, J. Alloys Compd., 2016, 656, 707–713 CrossRef CAS
.
- H. Liu, X. Liu, S. Wang, H.-K. Liu and L. Li, Energy Storage Mater., 2020, 28, 122–145 CrossRef
.
- S. Liu, D. Ni, H.-F. Li, K. N. Hui, C.-Y. Ouyang and S. C. Jun, J. Mater. Chem. A, 2018, 6, 10674–10685 RSC
.
- H. Adhikari, D. Neupane, C. K. Ranaweera, J. Candler, R. K. Gupta, S. Sapkota, X. Shen and S. R. Mishra, Electrochim. Acta, 2017, 225, 514–524 CrossRef CAS
.
- S. Al-Rubaye, R. Rajagopalan, C. M. Subramaniyam, Z. Yu, S. X. Dou and Z. Cheng, J. Power Sources, 2016, 324, 179–187 CrossRef CAS
.
- R. BoopathiRaja and M. Parthibavarman, J. Alloys Compd., 2019, 811, 152084 CrossRef CAS
.
- K. R. Shrestha, S. Kandula, N. H. Kim and J. H. Lee, J. Alloys Compd., 2019, 771, 810–820 CrossRef CAS
.
- Y. Zhang, L. Li, H. Su, W. Huang and X. Dong, J. Mater. Chem. A, 2015, 3, 43–59 RSC
.
- A. K. Mondal, D. Su, S. Chen, A. Ung, H.-S. Kim and G. Wang, Chem.–Eur. J., 2015, 21, 1526–1532 CrossRef CAS
.
- Y. Gao, Y. Xia, H. Wan, X. Xu and S. Jiang, Electrochim. Acta, 2019, 301, 294–303 CAS
.
- L.-B. Kong, C. Lu, M.-C. Liu, Y.-C. Luo, L. Kang, X. Li and F. C. Walsh, Electrochim. Acta, 2014, 115, 22–27 CrossRef CAS
.
- K. N. Hui, K. S. Hui, Z. Tang, V. V. Jadhav and Q. X. Xia, J. Power Sources, 2016, 330, 195–203 CrossRef CAS
.
- J. Xu, Y. Sun, M. Lu, L. Wang, J. Zhang, E. Tao, J. Qian and X. Liu, Acta Mater., 2018, 152, 162–174 CrossRef CAS
.
- V. Venkatachalam, A. Alsalme, A. Alghamdi and R. Jayavel, J. Electroanal. Chem., 2015, 756, 94–100 CrossRef CAS
.
- M. Li, W. Yang, J. Li, M. Feng, W. Li, H. Li and Y. Yu, Nanoscale, 2018, 10, 2218–2225 RSC
.
- J. Li, D. Xiong, L. Wang, M. K. S. Hirbod and X. Li, J. Energy Chem., 2019, 37, 66–72 CrossRef
.
- S. Nagamuthu, S. Vijayakumar, S.-H. Lee and K.-S. Ryu, Appl. Surf. Sci., 2016, 390, 202–208 CrossRef CAS
.
- S. Sahoo, K. K. Naik and C. S. Rout, Nanotechnology, 2015, 26, 455401 CrossRef
.
- Y. Dong, Y. Wang, Y. Xu, C. Chen, Y. Wang, L. Jiao and H. Yuan, Electrochim. Acta, 2017, 225, 39–46 CrossRef CAS
.
- M. A. Akhtar, V. Sharma, S. Biswas and A. Chandra, RSC Adv., 2016, 6, 96296–96305 RSC
.
- Z. Liu, F. Teng, C. Yuan, Z. Ul Abideen, W. Gu and Z. Liu, Energy Technol., 2019, 7, 1900314 CrossRef
.
- T. Huang, C. Zhao, L. Wu, X. Lang, K. Liu and Z. Hu, Ceram. Int., 2017, 43, 1968–1974 CrossRef CAS
.
- M. Shanmugavadivel, V. V. Dhayabaran and M. Subramanian, J. Phys. Chem. Solids, 2019, 133, 15–20 CrossRef CAS
.
- L. Li, Y. Q. Zhang, X. Y. Liu, S. J. Shi, X. Y. Zhao, H. Zhang, X. Ge, G. F. Cai, C. D. Gu, X. L. Wang and J. P. Tu, Electrochim. Acta, 2014, 116, 467–474 CrossRef CAS
.
- Y. Xu, X. Wang, C. An, Y. Wang, L. Jiao and H. Yuan, J. Mater. Chem. A, 2014, 2, 16480–16488 RSC
.
- Q. Wang, X. Wang, B. Liu, G. Yu, X. Hou, D. Chen and G. Shen, J. Mater. Chem. A, 2013, 1, 2468–2473 RSC
.
- L. Zhao, M. Yang, Z. Zhang, Y. Ji, Y. Teng, Y. Feng and X. Liu, Inorg. Chem. Commun., 2018, 89, 22–26 CrossRef CAS
.
- J.-J. Zhou, X. Han, K. Tao, Q. Li, Y.-L. Li, C. Chen and L. Han, Chem. Eng. J., 2018, 354, 875–884 CrossRef CAS
.
- Y. Lv, A. Liu, H. Che, J. Mu, Z. Guo, X. Zhang, Y. Bai, Z. Zhang, G. Wang and Z. Pei, Chem. Eng. J., 2018, 336, 64–73 CrossRef CAS
.
- T. Chen, R. Shi, Y. Zhang and Z. Wang, ChemPlusChem, 2019, 84, 69–77 CAS
.
- J. A.-A. Mehrez, K. A. Owusu, Q. Chen, L. Li, K. Hamwi, W. Luo and L. Mai, Inorg. Chem. Front., 2019, 6, 857–865 RSC
.
- K. R. Shrestha, S. Kandula, G. Rajeshkhanna, M. Srivastava, N. H. Kim and J. H. Lee, J. Mater. Chem. A, 2018, 6, 24509–24522 RSC
.
- S. Liu, K. S. Hui and K. N. Hui, ChemNanoMat, 2015, 1, 593–602 CrossRef CAS
.
- X. Zheng, Y. Ye, Q. Yang, B. Geng and X. Zhang, Dalton Trans., 2016, 45, 572–578 RSC
.
- Y. Zhang, H. Xuan, Y. Xu, B. Guo, H. Li, L. Kang, P. Han, D. Wang and Y. Du, Electrochim. Acta, 2016, 206, 278–290 CrossRef CAS
.
- M. Harilal, S. G. Krishnan, A. Yar, I. I. Misnon, M. V. Reddy, M. M. Yusoff, J. Ojur Dennis and R. Jose, J. Phys. Chem. C, 2017, 121, 21171–21183 CrossRef CAS
.
- X. Guo, M. Li, Y. Liu, Y. Huang, S. Geng, W. Yang and Y. Yu, J. Colloid Interface Sci., 2020, 563, 405–413 CrossRef CAS
.
- V. V. M. Chandu, Gopi, M. Venkata-Haritha, S.-K. Kim, K. Prabakar and H.-J. Kim, RSC Adv., 2016, 6, 102961–102967 RSC
.
- B. Cheng, W. Zhang, M. Yang, Y. Zhang and F. Meng, Ceram. Int., 2019, 45, 20451–20457 CrossRef CAS
.
- F. Liao, X. Han, Y. Zhang, X. Han, C. Xu and H. Chen, Ceram. Int., 2019, 45, 7244–7252 CrossRef CAS
.
- S. Liu, K. San Hui, K. N. Hui, J. M. Yun and K. H. Kim, J. Mater. Chem. A, 2016, 4, 8061–8071 RSC
.
- H.-M. Lee, C. V. V. Muralee Gopi, P. J. S. Rana, R. Vinodh, S. Kim, R. Padma and H.-J. Kim, New J. Chem., 2018, 42, 17190–17194 CAS
.
- Y. Feng, W. Liu, L. Sun, Y. Zhu, Y. Chen, M. Meng, J. Li, J. Yang, Y. Zhang and K. Liu, J. Alloys Compd., 2018, 753, 761–770 CAS
.
- L. Yu, G. Zhang, C. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137–139 CAS
.
- J. Yang, Y. Liu, S. Liu, L. Li, C. Zhang and T. Liu, Mater. Chem. Front., 2017, 1, 251–268 RSC
.
- F. Wang, X. Lv, L. Zhang, H. Zhang, Y. Zhu, Z. Hu, Y. Zhang, J. Ji and W. Jiang, J. Power Sources, 2018, 393, 169–176 CrossRef CAS
.
- P. Hu, D. Zhao, H. Liu, K. Chen and X. Wu, CrystEngComm, 2019, 21, 1600–1606 RSC
.
- S. Abouali, M. Akbari Garakani, Z.-L. Xu and J.-K. Kim, Carbon, 2016, 102, 262–272 CrossRef CAS
.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 CAS
.
- H. Wang, C. Shen, J. Liu, W. Zhang and S. Yao, J. Alloys Compd., 2019, 792, 122–129 CrossRef CAS
.
- D. Wu, H. Han, X. Hong, S. Tao, S. Xu, B. Qian, L. Wang, X. Chen and P. K. Chu, J. Alloys Compd., 2020, 846, 155720 CAS
.
- X. Hu, H. Nan, M. Liu, S. Liu, T. An and H. Tian, Electrochim. Acta, 2019, 306, 599–609 CrossRef CAS
.
- L. Li, F. He, S. Gai, S. Zhang, P. Gao, M. Zhang, Y. Chen and P. Yang, CrystEngComm, 2014, 16, 9873–9881 RSC
.
- T. T. Gebremariam, F. Chen, Q. Wang, J. Wang, Y. Liu, X. Wang and A. Qaseem, ACS Appl. Energy Mater., 2018, 1, 1612–1625 CrossRef CAS
.
- P. Hao, Z. Zhao, L. Li, C.-C. Tuan, H. Li, Y. Sang, H. Jiang, C. P. Wong and H. Liu, Nanoscale, 2015, 7, 14401–14412 RSC
.
- T. Pettong, P. Iamprasertkun, A. Krittayavathananon, P. Sukha, P. Sirisinudomkit, A. Seubsai, M. Chareonpanich, P. Kongkachuichay, J. Limtrakul and M. Sawangphruk, ACS Appl. Mater. Interfaces, 2016, 8, 34045–34053 CrossRef CAS
.
- Y. Zhu, C. Zhang, S. Tang, H. Huang, S. Wang, Q. Luo and Y. Du, ACS Appl. Energy Mater., 2019, 2, 7546–7553 CrossRef CAS
.
- M. Zhang, W. Liu, R. Liang, R. Tjandra and A. Yu, Sustainable Energy Fuels, 2019, 3, 2499–2508 RSC
.
- P. Saren, A. De Adhikari, S. Khan and G. C. Nayak, J. Solid State Chem., 2019, 271, 282–291 CAS
.
- G. Liu, B. Wang, T. Liu, L. Wang, H. Luo, T. Gao, F. Wang, A. Liu and D. Wang, J. Mater. Chem. A, 2018, 6, 1822–1831 RSC
.
- S. G. Mohamed, T.-F. Hung, C.-J. Chen, C. K. Chen, S.-F. Hu and R.-S. Liu, RSC Adv., 2014, 4, 17230–17235 RSC
.
- C. Xiao, X. Zhang and D. R. MacFarlane, Electrochim. Acta, 2018, 280, 55–61 CrossRef CAS
.
- N. Cai, J. Fu, V. Chan, M. Liu, W. Chen, J. Wang, H. Zeng and F. Yu, J. Alloys Compd., 2019, 782, 251–262 CrossRef CAS
.
- Y. Ji, J. Xie, J. Wu, Y. Yang, X.-Z. Fu, R. Sun and C.-P. Wong, J. Power Sources, 2018, 393, 54–61 CrossRef CAS
.
- H. Che, Y. Lv, A. Liu, H. Li, Z. Guo, J. Mu, Y. Wang and X. Zhang, Chem. Eng. J., 2020, 384, 123372 CrossRef CAS
.
- Y. Zhao, L. Hu, S. Zhao and L. Wu, Adv. Funct. Mater., 2016, 26, 4085–4093 CrossRef CAS
.
- Y. Lv, Z. Guo, A. Liu, H. Che, J. Mu, X. Zhang, Y. Bai, Z. Zhang and G. Wang, Ceram. Int., 2017, 43, 12948–12956 CrossRef CAS
.
- Y. Lv, A. Liu, Z. Shi, J. Mu, Z. Guo, X. Zhang and H. Che, ChemElectroChem, 2018, 5, 3968–3979 CAS
.
- W. Li, K. Xu, G. Song, X. Zhou, R. Zou, J. Yang, Z. Chen and J. Hu, CrystEngComm, 2014, 16, 2335–2339 RSC
.
- A. Krittayavathananon, T. Pettong, P. Kidkhunthod and M. Sawangphruk, Electrochim. Acta, 2017, 258, 1008–1015 CrossRef CAS
.
- S. Li, J. Wang, M. Wang and Y. Ni, CrystEngComm, 2019, 21, 403–410 RSC
.
- H. Che, A. Liu, J. Mu, C. Wu and X. Zhang, Ceram. Int., 2016, 42, 2416–2424 CrossRef CAS
.
- H. Che, Y. Wang and Y. Mao, J. Alloys Compd., 2016, 680, 586–594 CrossRef CAS
.
- M. Haripriya, A. M. Ashok, S. Hussain and R. Sivasubramanian, Ionics, 2020 DOI:10.1007/s11581-020-03788-y
.
- H. Liu, Z. Guo, S. Wang, X. Xun, D. Chen and J. Lian, J. Alloys Compd., 2020, 846, 156504 CAS
.
- Y. Zhai, H. Mao, P. Liu, X. Ren, L. Xu and Y. Qian, J. Mater. Chem. A, 2015, 3, 16142–16149 RSC
.
- N. Mohamed and N. K. Allam, RSC Adv., 2020, 10, 21662–21685 RSC
.
- M. M. S. Sanad, A. K. Yousef, M. M. Rashad, A. H. Naggar and A. Y. El-Sayed, Phys. B, 2020, 579, 411889 CrossRef CAS
.
- X. Wu, S. Li, B. Wang, J. Liu and M. Yu, New J. Chem., 2015, 39, 8416–8423 RSC
.
- C. Fu, G. Li, D. Luo, X. Huang, J. Zheng and L. Li, ACS Appl. Mater. Interfaces, 2014, 6, 2439–2449 CrossRef CAS
.
- D. Darbar, M. R. Anilkumar, V. Rajagopalan, I. Bhattacharya, H. I. Elim, T. Ramakrishnappa, F. I. Ezema, R. Jose and M. V. Reddy, Ceram. Int., 2018, 44, 4630–4639 CrossRef CAS
.
- S. Zhou, X. Luo, L. Chen, C. Xu and D. Yan, Ceram. Int., 2018, 44, 17858–17863 CAS
.
- Y. Zhang, X. Wang, Q. Zhao, Y. Fu, H. Wang and H. Shu, Electrochim. Acta, 2015, 180, 866–872 CrossRef CAS
.
- L. Zhang, Q. Tang, X. Chen, B. Fan, K. Xiao, S. Zhang, W. Deng and A. Hu, J. Alloys Compd., 2017, 722, 387–393 CrossRef CAS
.
- M. V. Reddy, Y. Xu, V. Rajarajan, T. Ouyang and B. V. R. Chowdari, ACS Sustainable Chem. Eng., 2015, 3, 3035–3042 CrossRef CAS
.
- G. Li, Q. Zhai, Q. Liu and R. Jin, Cryst. Res. Technol., 2017, 52, 1600255 CrossRef
.
- J. Qi, P. Wang, Y. Yan, X. Zheng, J. Cao and J. Feng, Vacuum, 2019, 169, 108959 CrossRef CAS
.
- D. S. Baji, H. S. Jadhav, S. V. Nair and A. K. Rai, J. Solid State Chem., 2018, 262, 191–198 CrossRef CAS
.
- G. Li, L. Xu, Y. Zhai and Y. Hou, J. Mater. Chem. A, 2015, 3, 14298–14306 RSC
.
- X. Hou, X. Wang, B. Liu, Q. Wang, T. Luo, D. Chen and G. Shen, Nanoscale, 2014, 6, 8858–8864 RSC
.
- T. Huang, Z. Lou, Y. Lu, R. Li, Y. Jiang, G. Shen and D. Chen, ChemElectroChem, 2019, 6, 5836–5843 CrossRef CAS
.
- L. Ni, W. Tang, X. Liu, N. Zhang, J. Wang, S. Liang, R. Ma and G. Qiu, Dalton Trans., 2018, 47, 3775–3784 RSC
.
- F. Yang, W. Li and B. Tang, Chem. Eng. J., 2018, 334, 2021–2029 CrossRef CAS
.
- J. Li, J. Wang, X. Liang, Z. Zhang, H. Liu, Y. Qian and S. Xiong, ACS Appl. Mater. Interfaces, 2014, 6, 24–30 CrossRef CAS
.
- G. Huang, S. Xu, Y. Yang, H. Sun and Z. Xu, RSC Adv., 2016, 6, 10763–10774 RSC
.
- R. Jin, Y. Ma, Y. Sun, H. Li, Q. Wang and G. Chen, Energy Technol., 2017, 5, 293–299 CrossRef CAS
.
- J. Li, S. Xiong, X. Li and Y. Qian, Nanoscale, 2013, 5, 2045–2054 RSC
.
- H. Yang, Y. Xie, M. Zhu, Y. Liu, Z. Wang, M. Xu and S. Lin, Dalton Trans., 2019, 48, 9205–9213 RSC
.
- Y. Jin, L. Wang, Q. Jiang, X. Du, C. Ji and X. He, Mater. Lett., 2016, 177, 85–88 CrossRef CAS
.
- H. Xu, H. Shen, X. Song, X. Kong, Y. Zhang and Z. Qin, J. Electroanal. Chem., 2019, 851, 113455 CrossRef CAS
.
- X. Kong, T. Zhu, F. Cheng, M. Zhu, X. Cao, S. Liang, G. Cao and A. Pan, ACS Appl. Mater. Interfaces, 2018, 10, 8730–8738 CrossRef CAS
.
- G. Huang, S. Xu, Z. Xu, H. Sun and L. Li, ACS Appl. Mater. Interfaces, 2014, 6, 21325–21334 CrossRef CAS
.
- J. Zhu, G. Cao, Y. Zhou, Y. Li, J. Zheng and D. Zhang, ChemSusChem, 2020, 13, 1890–1899 CrossRef CAS
.
- T. Li, X. Li, Z. Wang, H. Guo, Q. Hu and W. Peng, Electrochim. Acta, 2016, 191, 392–400 CrossRef CAS
.
- M. Wang, X. Yu, L. Hou, A. Gagnoud, Y. Fautrelle, R. Moreau and X. Li, Chem. Eng. J., 2018, 351, 930–938 CrossRef CAS
.
- A. F. Shaikh, R. S. Kalubarme, M. S. Tamboli, S. S. Patil, M. V. Kulkarni, D. R. Patil, S. W. Gosavi, C.-J. Park and B. B. Kale, ChemistrySelect, 2017, 2, 4630–4637 CrossRef CAS
.
- L. Wu, J. Lang, S. Wang, P. Zhang and X. Yan, Electrochim. Acta, 2016, 203, 128–135 CrossRef CAS
.
- Y. Zhu, Y. Huang and M. Wang, Chem. Eng. J., 2019, 378, 122207 CrossRef CAS
.
- R. Ding, Z. Li, C. Wang and M. Chen, J. Alloys Compd., 2017, 726, 445–452 CrossRef CAS
.
- P. Huang, M. Zhao, B. Jin, H. Li, Z. Zhu, L. Jiang and Q. Jiang, Dalton Trans., 2018, 47, 14540–14548 RSC
.
- P. Zhang, J. Liu, J. Wu, W. Wang, C. Zhou, S. Guo, S. Li, Y. Yang and L. Chen, Mater. Today Energy, 2020, 17, 100451 CrossRef
.
- C. Chen, B. Liu, Q. Ru, S. Ma, B. An, X. Hou and S. Hu, J. Power Sources, 2016, 326, 252–263 CrossRef CAS
.
- G. Huang, X. Guo, X. Cao, Q. Tian and H. Sun, J. Alloys Compd., 2017, 695, 2937–2944 CrossRef CAS
.
- P. Kulkarni, D. Ghosh, G. Balakrishna, R. S. Rawat, S. Adams and M. V. Reddy, Ceram. Int., 2019, 45, 10619–10625 CrossRef CAS
.
- R. Jin, Y. Meng, Y. Ma, H. Li, Y. Sun and G. Chen, Electrochim. Acta, 2016, 209, 163–170 CrossRef CAS
.
- L.-Q. Fan, J.-L. Huang, Y.-L. Wang, C.-L. Geng, S.-J. Sun, Y.-F. Huang and J.-H. Wu, J. Energy Storage, 2020, 30, 101427 CrossRef
.
- P. Huang, F. Xu, G. Zhu, C. Dong, B. Jin, H. Li and Q. Jiang, ChemPlusChem, 2019, 84, 1596–1603 CrossRef CAS
.
- L. Wu, J. Lang, P. Zhang, X. Zhang, R. Guo and X. Yan, J. Mater. Chem. A, 2016, 4, 18392–18400 RSC
.
- X. Wu, W. Wu, K. Wang, W. Chen and D. He, Mater. Lett., 2015, 147, 85–87 CrossRef CAS
.
- M. Islam, M. Akbar, G. Ali, K.-W. Nam, K. Y. Chung and H.-G. Jung, Ceram. Int., 2020, 46, 26147–26155 CrossRef CAS
.
- L. Zhu, F. Li, T. Yao, T. Liu, J. Wang, Y. Li, H. Lu, R. Qian, Y. Liu and H. Wang, Energy Fuels, 2020, 34, 11574–11580 CrossRef CAS
.
- L. Hou, S. Deng, Y. Jiang, R. Cui, Y. Zhou, Y. Guo, J. Li and F. Gao, Nanotechnology, 2020, 31, 375404 CrossRef CAS
.
- M. A. Rahman, X. Wang and C. Wen, J. Appl. Electrochem., 2014, 44, 5–22 CrossRef CAS
.
- R. S. Kalubarme, H. S. Jadhav, D. T. Ngo, G.-E. Park, J. G. Fisher, Y.-I. Choi, W.-H. Ryu and C.-J. Park, Sci. Rep., 2015, 5, 13266 CrossRef CAS
.
- S. G. Mohamed, Y.-Q. Tsai, C.-J. Chen, Y.-T. Tsai, T.-F. Hung, W.-S. Chang and R.-S. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 12038–12046 CrossRef CAS
.
- K. Song, L. Yuan, Z. Li, Y. Lv, B. Yang, Y. Yu, X. Shen and X. Hu, Electrochim. Acta, 2020, 353, 136572 CrossRef CAS
.
- H. Wu, W. Sun, Y. Wang, F. Wang, J. Liu, X. Yue, Z. Wang, J. Qiao, D. W. Rooney and K. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 12355–12365 CrossRef CAS
.
- H. Wu, W. Sun, J. Shen, C. Lu, Y. Wang, Z. Wang and K. Sun, Nanoscale, 2018, 10, 13149–13158 RSC
.
- L. Zou, J. Cheng, Y. Jiang, Y. Gong, B. Chi, J. Pu and L. Jian, RSC Adv., 2016, 6, 31248–31255 RSC
.
- S. Ma, L. Sun, L. Cong, X. Gao, C. Yao, X. Guo, L. Tai, P. Mei, Y. Zeng, H. Xie and R. Wang, J. Phys. Chem. C, 2013, 117, 25890–25897 CrossRef CAS
.
- X. Cao, J. Wu, C. Jin, J. Tian, P. Strasser and R. Yang, ACS Catal., 2015, 5, 4890–4896 CrossRef CAS
.
- X. Cao, Z. Sun, X. Zheng, J. Tian, C. Jin, R. Yang, F. Li, P. He and H. Zhou, J. Mater. Chem. A, 2017, 5, 19991–19996 RSC
.
- X. Cao, Z. Sun, X. Zheng, C. Jin, J. Tian, X. Li and R. Yang, ChemSusChem, 2018, 11, 574–579 CrossRef CAS
.
- Z. Li, Y. Lv, Y. Yu, J. Yin, K. Song, B. Yang, L. Yuan and X. Hu, J. Alloys Compd., 2020, 817, 152736 CrossRef CAS
.
- G. Karkera, S. G. Chandrappa and A. S. Prakash, Chem.–Eur. J., 2018, 24, 17303–17310 CrossRef CAS
.
- H. Wang, Y. Yang, Y. Liang, G. Zheng, Y. Li, Y. Cui and H. Dai, Energy Environ. Sci., 2012, 5, 7931–7935 RSC
.
- J. G. Kim, Y. Kim, Y. Noh and W. B. Kim, ChemSusChem, 2015, 8, 1752–1760 CrossRef CAS
.
- F. Zhu, J. Zhang, B. Yang, X. Shi, C. Lu, J. Yin, Y. Yu and X. Hu, J. Alloys Compd., 2018, 749, 433–440 CrossRef CAS
.
- T. Ishihara, K. Yokoe, T. Miyano and H. Kusaba, Electrochim. Acta, 2019, 300, 455–460 CrossRef CAS
.
- S. G. Chandrappa, P. Moni, G. Karkera and A. S. Prakash, Nanoscale Adv., 2019, 1, 2392–2399 RSC
.
- C. Shenghai, S. Liping, K. Fanhao, H. Lihua and Z. Hui, J. Power Sources, 2019, 430, 25–31 CrossRef CAS
.
- K.-N. Jung, S. M. Hwang, M.-S. Park, K. J. Kim, J.-G. Kim, S. X. Dou, J. H. Kim and J.-W. Lee, Sci. Rep., 2015, 5, 7665 CrossRef CAS
.
- D. Bin, Z. Guo, A. G. Tamirat, Y. Ma, Y. Wang and Y. Xia, Nanoscale, 2017, 9, 11148–11157 RSC
.
- Y. Kang, D. Zou, J. Zhang, F. Liang, K. Hayashi, H. Wang, D. Xue, K. Chen, K. R. Adair and X. Sun, Electrochim. Acta, 2017, 244, 222–229 CrossRef CAS
.
- Y. Liu, X. Chi, Q. Han, Y. Du, J. Huang, X. Lin and Y. Liu, Nanoscale, 2019, 11, 5285–5294 RSC
.
- Y. Zhu, X. Liu, S. Jin, H. Chen, W. Lee, M. Liu and Y. Chen, J. Mater. Chem. A, 2019, 7, 5875–5897 RSC
.
- F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC
.
- M. Shao, Q. Chang, J.-P. Dodelet and R. Chenitz, Chem. Rev., 2016, 116, 3594–3657 CrossRef CAS
.
- Y. Wang, Q. Cao, C. Guan and C. Cheng, Small, 2020, 16, 2002902 CrossRef CAS
.
- J. Han, J. Bian and C. Sun, Research, 2020, 2020, 9512763 CAS
.
- T. Zhao, S. Gadipelli, G. He, M. J. Ward, D. Do, P. Zhang and Z. Guo, Chemsuschem, 2018, 11, 1295–1304 CrossRef CAS
.
- D. Yan, Y. Li, J. Huo, R. Chen, L. Dai and S. Wang, Adv. Mater., 2017, 29, 1606459 CrossRef
.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC
.
- H. Yang, F. Hu, Y. Zhang, L. Shi and Q. Wang, Nano Res., 2016, 9, 207–213 CrossRef CAS
.
- T. Y. Ma, Y. Zheng, S. Dai, M. Jaroniec and S. Z. Qiao, J. Mater. Chem. A, 2014, 2, 8676–8682 RSC
.
- W. Wang, L. Kuai, W. Cao, M. Huttula, S. Ollikkala, T. Ahopelto, A.-P. Honkanen, S. Huotari, M. Yu and B. Geng, Angew. Chem., Int. Ed., 2017, 56, 14977–14981 CrossRef CAS
.
- G. Fu, Z. Liu, J. Zhang, J. Wu, L. Xu, D. Sun, J. Zhang, Y. Tang and P. Chen, Nano Res., 2016, 9, 2110–2122 CrossRef CAS
.
- P. W. Menezes, A. Indra, N. R. Sahraie, A. Bergmann, P. Strasser and M. Driess, Chemsuschem, 2015, 8, 164–171 CrossRef CAS
.
- T. Zhang, Z. Li, L. Wang, P. Sun, Z. Zhang and S. Wang, Chemsuschem, 2018, 11, 2730–2736 CrossRef CAS
.
- W. Fu, X.-L. Wang, X.-X. Yang and X.-Q. He, ChemistrySelect, 2018, 3, 4228–4236 CrossRef CAS
.
- X. He, F. Yin, S. Yuan, N. Liu and X. Huang, Chemelectrochem, 2016, 3, 1107–1115 CrossRef CAS
.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517–3523 CrossRef CAS
.
- X. Cao, C. Jin, F. Lu, Z. Yang, M. Shen and R. Yang, J. Electrochem. Soc., 2014, 161, H296–H300 CrossRef CAS
.
- J. Shi, K. Lei, W. Sun, F. Li, F. Cheng and J. Chen, Nano Res., 2017, 10, 3836–3847 CrossRef CAS
.
- X. Chen, R. Li, J. Wang, Q. Zhong and Y. Bu, ChemistrySelect, 2016, 1, 2159–2162 CrossRef CAS
.
- S. K. Singh, V. Kashyap, N. Manna, S. N. Bhange, R. Soni, R. Boukherroub, S. Szunerits and S. Kurungot, ACS Catal., 2017, 7, 6700–6710 CrossRef CAS
.
- A. Ashok, A. Kumar, J. Ponraj and S. A. Mansour, J. Electrochem. Soc., 2020, 167, 054507 CrossRef CAS
.
- X. Ge, Y. Liu, F. W. T. Goh, T. S. A. Hor, Y. Zong, P. Xiao, Z. Zhang, S. H. Lim, B. Li, X. Wang and Z. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 12684–12691 CrossRef CAS
.
- A. Zhao, J. Masa, W. Xia, A. Maljusch, M.-G. Willinger, G. Clavel, K. Xie, R. Schlögl, W. Schuhmann and M. Muhler, J. Am. Chem. Soc., 2014, 136, 7551–7554 CrossRef CAS
.
- Y. Yang, R. Zeng, Y. Xiong, F. J. DiSalvo and H. D. Abruña, Chem. Mater., 2019, 31, 9331–9337 CrossRef CAS
.
- C. Xu, M. Lu, Y. Zhan and J. Y. Lee, RSC Adv., 2014, 4, 25089–25092 RSC
.
- X. Yan, Y. Jia, J. Chen, Z. Zhu and X. Yao, Adv. Mater., 2016, 28, 8771–8778 CrossRef CAS
.
- D. Wang, X. Chen, D. G. Evans and W. Yang, Nanoscale, 2013, 5, 5312–5315 RSC
.
- J. M. Gonçalves, M. Ireno da Silva, L. Angnes and K. Araki, J. Mater. Chem. A, 2020, 8, 2171–2206 RSC
.
- C. Ye, M. Q. Wang, S. J. Bao and C. Ye, ACS Appl. Mater. Interfaces, 2019, 11, 30887–30893 CrossRef CAS
.
- L. Yu, H. Zhou, J. Sun, F. Qin, F. Yu, J. Bao, Y. Yu, S. Chen and Z. Ren, Energy Environ. Sci., 2017, 10, 1820–1827 RSC
.
- Y. Gong, Z. Xu, H. Pan, Y. Lin, Z. Yang and X. Du, J. Mater. Chem. A, 2018, 6, 5098–5106 RSC
.
- J. M. Gonçalves, P. R. Martins, K. Araki and L. Angnes, J. Mater. Chem. A, 2020, 8, 2171–2206 RSC
.
- S. Chu, H. Sun, G. Chen, Y. Chen, W. Zhou and Z. Shao, ACS Appl. Mater. Interfaces, 2019, 11, 25227–25235 CrossRef CAS
.
- Y.-J. Wang, H. Fan, A. Ignaszak, L. Zhang, S. Shao, D. P. Wilkinson and J. Zhang, Chem. Eng. J., 2018, 348, 416–437 CrossRef CAS
.
- Y. Gong, Y. Lin, Z. Yang, F. Jiao, J. Li and W. Wang, Appl. Surf. Sci., 2019, 476, 840–849 CrossRef CAS
.
- J. Zhang, X. Zhao, L. Du, Y. Li, L. Zhang, S. Liao, J. B. Goodenough and Z. Cui, Nano Lett., 2019, 19, 7457–7463 CrossRef CAS
.
- J. Béjar, L. Álvarez-Contreras, J. Ledesma-García, N. Arjona and L. G. Arriaga, J. Electroanal. Chem., 2019, 847, 113190 CrossRef
.
- Y. Huang, W. Yang, Y. Yu and S. Hao, J. Electroanal. Chem., 2019, 840, 409–414 CrossRef CAS
.
- A. Rebekah, E. Ashok Kumar, C. Viswanathan and N. Ponpandian, Int. J. Hydrogen Energy, 2020, 45, 6391–6403 CrossRef CAS
.
- A. Rebekah, S. Anantharaj, C. Viswanthan and N. Ponpandian, Int. J. Hydrogen Energy, 2020, 45, 14713–14727 CrossRef CAS
.
- X. Huang, H. Zheng, G. Lu, P. Wang, L. Xing, J. Wang and G. Wang, ACS Sustainable Chem. Eng., 2019, 7, 1169–1177 CrossRef CAS
.
- R. Burch, Phys. Chem. Chem. Phys., 2006, 8, 5483–5500 RSC
.
- Y. Suchorski, R. Wrobel, S. Becker and H. Weiss, J. Phys. Chem. C, 2008, 112, 20012–20017 CrossRef CAS
.
- F. Yang, J. Graciani, J. Evans, P. Liu, J. Hrbek, J. F. Sanz and J. A. Rodriguez, J. Am. Chem. Soc., 2011, 133, 3444–3451 CrossRef CAS
.
- X. Du, H. Su and X. Zhang, Int. J. Hydrogen Energy, 2019, 44, 21637–21650 CrossRef CAS
.
- C. Sun, J. Yang, Z. Dai, X. Wang, Y. Zhang, L. Li, P. Chen, W. Huang and X. Dong, Nano Res., 2016, 9, 1300–1309 CrossRef CAS
.
- M. Liu, R. Zhang and W. Chen, Chem. Rev., 2014, 114, 5117–5160 CrossRef CAS
.
- S. Hirai, S. Yagi, A. Seno, M. Fujioka, T. Ohno and T. Matsuda, RSC Adv., 2016, 6, 2019–2023 RSC
.
- J. Ge, W. Zhang, J. Tu, T. Xia, S. Chen and G. Xie, Small, 2020, 16, 2001856 CrossRef CAS
.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS
.
- K. Wang, X. She, S. Chen, H. Liu, D. Li, Y. Wang, H. Zhang, D. Yang and X. Yao, J. Mater. Chem. A, 2018, 6, 5560–5565 RSC
.
- J. Han, S. Hao, Z. Liu, A. M. Asiri, X. Sun and Y. Xu, Chem. Commun., 2018, 54, 1077–1080 RSC
.
- J. Li, Y. Zhang, L. Li, Y. Wang, L. Zhang, B. Zhang, F. Wang, B. Li and X.-Y. Yu, Dalton Trans., 2019, 48, 17022–17028 RSC
.
- X. Du, J. Fu and X. Zhang, CrystEngComm, 2019, 21, 7293–7302 RSC
.
- K. Lankauf, K. Cysewska, J. Karczewski, A. Mielewczyk-Gryń, K. Górnicka, G. Cempura, M. Chen, P. Jasiński and S. Molin, Int. J. Hydrogen Energy, 2020, 45, 14867–14879 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2021 |






